Abstract
Background
Peculiar erythema known as annular erythema associated with Sjögren's syndrome (AESS) can be differentiated from autoimmune annular erythema and subacute cutaneous lupus erythematosus, both clinically and histologically. However, there are no detailed investigations on immune competent cells infiltration.
Objective
Preferential infiltration of interleukin-17-producing T helper (Th17) cells and regulatory T (Treg) cells into the labial salivary gland is reported to play a role in maintaining mucoepithelitis in patients with Sjögren's syndrome. In this study, we evaluated Th17 and Treg cell infiltration into the lesional skin of AESS.
Methods
We analyzed the numbers and infiltration patterns of Th17 and FoxP3 (+) Treg cells in seven cases of AESS using immunohistochemistry. Seven patients with systemic lupus erythematosus (SLE), atopic dermatitis (AD) and psoriasis vulgaris (PV), which are representatives of Th17 cell-involved skin disorders, were enrolled as disease controls.
Results
Periappendageal and epidermal changes, such as follicular plugging and liquefaction, were evident in the annular erythema of SLE, not AESS, tissue samples. In AESS tissue samples, dense perivascular and periappendageal infiltration of lymph cells was observed in the middle-to-deep dermis, as previously described, in contrast to the superficial infiltration pattern observed in both AD and PV samples. While the total number of infiltrated lymphocytes was similar between AESS and SLE tissue samples, Th17 cells were found to be preferentially infiltrated in the middle-to-deep dermis in AESS samples.
Annular erythema has been recognized to be a specific, cutaneous manifestation of Sjögren's syndrome (SS). In 1989, we reported four patients with primary SS who presented with distinct annular erythema characterized by a wide, elevated border and central pallor1. This peculiar erythema, which is also known as annular erythema associated with Sjögren's Syndrome (AESS), can be differentiated from autoimmune annular erythema and subacute cutaneous lupus erythematosus (SCLE), both clinically and histologically1,2,3,4. Based on the literature reported since 2007, this type of erythema preferentially occurs in Oriental, but not Occidental, populations1,4. At present, three clinical types are known: isolated donut ring-like erythema with an elevated border (type I), SCLE-like marginally scaled polycyclic erythema (type II) and papular insect bite-like erythema (type III), all of which exhibit the common histological characteristics described above and meet the diagnostic criteria for SS.
Histologically, AESS is characterized by coat sleeve-like infiltration of lymphocytes around blood vessels and nuclear debris in connective tissues. In the present study, no vasculitis or epidermal changes suggestive of lupus erythematosus were observed in any of the cases, although immunoglobulin and/or complement deposition along the basement membrane zone and focal liquefaction degeneration of the basal layer in the involved skin were seen in some cases. Generally, the major dermal infiltrates consist primarily of CD4 (+) and 4B4 (+) (CD45RO) lymphocytes3. Although recent studies have documented that Th17 cells5,6,7 and regulatory T (Treg) cells8,9 preferentially infiltrate into the labial salivary gland in patients with SS, there has been no detailed investigation of this type of cell infiltration in patients with AESS. In this study, seven patients with AESS were enrolled to evaluate infiltration of Th17 cells and their counterpart Treg cells in the lesional skin of AESS compared with that observed in other skin diseases, such as atopic dermatitis (AD), psoriasis vulgaris (PV) and systemic lupus erythematosus (SLE).
The clinical characteristics of the seven patients with AESS are described in Table 1. Seven patients with SLE, AD and PV were also enrolled as disease controls. All patients fulfilled the diagnostic criteria for AD10, SS11 and SLE12. All patients with SS exhibited moderate-to-severe, persistent itchy skin lesions and Sicca symptoms, including dry eyes and dry mouth with moderate joint pain. Biopsied specimens were obtained under informed consent to make a correct diagnosis of annular erythema.
Paraffin-embedded biopsy tissues were subjected to hematoxylin and eosin staining as well as immunohistochemical staining using mouse anti-human CD3 antibodies (Dako-Cytomation, Carpinteria, CA, USA), anti-human Foxp3 antibodies (Abcam, Cambridge, UK) and anti-human interleukin (IL)-17A antibodies (R&D Systems, Minneapolis, MN, USA) to evaluate the number of infiltrating T lymphocytes, Treg cells and Th17 cells using an enzyme-labeled antibody method. Since IL-17A (+) cells were also positive for CD3, not CD8, and no γδT cells were observed (data not shown), we regarded the IL-17A (+) cells to be Th17 cells. As a brief explanation of staining, phosphate buffered saline supplemented with 2% bovine serum albumin was used as a blocking reagent, a 1:50 dilution of the antibodies was used as the primary antibody and a peroxidase-conjugated goat anti-mouse/rabbit immunoglobulin antibody (Dako-Cytomation) was used as the secondary antibody. The positive cells were colorized us alkalinephosphatase. The numbers of CD3 T-cells, Th17 cells and FoxP3 (+) Treg cells in the superficial (upper 1/3 of the dermis) and middle-to-deep (lower 2/3 of the dermis) dermis were counted on three non-contiguous random grids under high-power field (×200) microscopy. The results are expressed as the number of positive cells per field, and the ratios of Th17 cells to CD3 T-cells and FoxP3 (+) T-cells to CD3 T-cells were calculated and expressed as the mean±standard deviation (SD).
The data are expressed as the mean value±SD. The Mann-Whitney U-test was used to determine the level of significance between the samples. A p-value of less than 0.05 was considered to be significant. All of the statistical analyse were performed using The Prism software version 5 (GraphPad Software Inc., San Diego, CA, USA).
In the AESS tissue samples, dense perivascular and periappendageal infiltration of mononuclear cells was observed as well as previously described, contrary to the quite superficial infiltration pattern observed in the AD and PV tissue samples (Fig. 1). While the total number of infiltrated lymphocytes was similar between the AESS and SLE tissue samples, the number of Th17 cells located in the middle-to-deep dermis was higher in the AESS samples than that in the SLE samples (Fig. 2). The numbers of Th17 cells and Treg cells in the middle-to-deep dermis in the AESS samples were significantly higher than those observed in the AD and PV samples. Infiltration of these cells in the PV samples was prominent in the superficial dermis (Fig. 2). The ratios of Treg or Th17 cells to T lymphocytes in the middle-to-deep dermis did not differ between the AESS and SLE samples and were significantly higher in the AESS and SLE samples than in the PV and AD samples (Fig. 3). These results suggest that an increased amount of Th17 cell infiltration is a coincident feature of annular lesions and mucoepithelitis (Fig. 4) in patients with SS, as reported in recent reports5,6,7.
AESS is recognized to be a specific, cutaneous manifestation of SS. In 1989, four patients with primary SS presented with distinct annular erythema characterized by a wide, elevated border (likened to a doughnut ring) and central pallor1. This peculiar erythema, designated AESS, can be clinically and histologically differentiated from both autoimmune annular erythema and SCLE. We previously reported that most of the infiltrating cells observed in AESS are CD4 (+), CD45RO (+) T-cells13. These cells express the oligoclonal T-cell receptor Vβ 2/13, produce IL-6, interferon-γ and tissue growth factor-β and disrupt epithelial cells through the FAS/FAS-ligand (FAS-L) system, as reported in previous studies14,15. Minor populations of CD8 (+) T-cells are speculated to injure salivary glands due to perforin/granzyme production16. Tissue-specific autoantibodies, such as anti-α-fodrin antibodies or anti-muscarinic M3 receptor antibodies, are also thought to play a role in the induction of mucoepithelitis in patients with SS17.
AESS preferentially occurs in Asian, not Western populations; immunogenetic analysis by Miyagawa et al.18 suggested that SS-associated erythema exhibits a close association with HLA-DRw52, a relatively common locus among Orientals. The involvement of Th17 cells in SS5,6,7, PV19,20 and AD21 in addition to the Th1 and Th2 balance theory is thought to be responsible for the development of systemic autoimmune diseases and common skin diseases, such as AD and PV. In the present study, the numbers and percentages of Th17 and FoxP3 (+) T-cells were similar between the AESS and SLE samples, although there were some differences in the infiltration patterns observed between these two diseases. This finding may be attributable to the heterogeneity of SLE (acute LE to chronic LE)2 in contrast to the rather homogenous manifestations of AESS. A Japanese report documented cases transitioning from type I AESS to SCLE with epidermal changes and histological LE22 development during the clinical course, suggesting that common etiopathological factors may be involved in the pathogenesis of SCLE and AESS, as proposed by McCauliffe et al.22, Ruzicka et al.23, and Provost et al.24. Therefore, we postulate that transient cases may exist, not only among Occidental, but also among Asian patients with positive anti-SSA antibodies.
These results suggest that an increased number and distribution of infiltration of Th17 cells in lesional skin is a preferential histopathological feature of AESS rather than SLE and is associated with the concomitant occurrence of mucoepithelitis in SS patients.
Figures and Tables
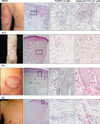 | Fig. 1Clinical and histopathological features of AESS, SLE, PV and AD patients. Histopathology at low magnification (immunohistochemical stain, ×20) is placed on the 2nd column and that at high magnification (immunohistochemical stain, ×100) is placed on the 3rd and 4th columns. The bar indicates 50 µm. AESS: annular erythema associated with Sjögren's syndrome, SLE: systemic lupus erythematosus, PV: psoriasis vulgaris, AD: atopic dermatitis. |
 | Fig. 2Total numbers of CD3 (+) T-cells, Th17 cells and FoxP3 (+) T-cells in the upper dermis (A) and middle-to-deep dermis (B) of the AESS, SLE, PV, and AD tissue samples. Increased numbers of Th17 cells and FoxP3 (+) Treg cells in the middle-to-deep dermis were observed in the AESS and SLE samples compared to that seen in the PV and AD samples. The bars indicate the mean±standard deviation. p-values of <0.05 and <0.01 are represented by * and **, respectively. HPF: high power field, AESS: annular erythema associated with Sjögren's syndrome, SLE: systemic lupus erythematosus, PV: psoriasis vulgaris, AD: atopic dermatitis. |
 | Fig. 3Infiltration patterns of CD3 (+) T-cells, Th17 cells and FoxP3 (+) T-cells in the AESS, SLE, PV and AD samples. The percentages of the cells were similar in the superficial (A) and middle-to-deep dermis (B) of the AESS and SLE samples. The bars indicate the mean±standard deviation. p-values of <0.05 and <0.01 are represented by * and **, respectively. AESS: annular erythema associated with Sjögren's syndrome, SLE: systemic lupus erythematosus, PV: psoriasis vulgaris, AD: atopic dermatitis, IL: interleukin. |
ACKNOWLEDGMENT
We thank Kenju Nishida, Eriko Nobuyoshi and Sayaka Matsumura for their significant technical assistance.
References
1. Teramoto N, Katayama I, Arai H, Eto H, Kamimura K, Uetsuka M, et al. Annular erythema: a possible association with primary Sjögren's syndrome. J Am Acad Dermatol. 1989; 20:596–601.

2. Katayama I, Teramoto N, Arai H, Nishioka K, Nishiyama S. Annular erythema. A comparative study of Sjögren syndrome with subacute cutaneous lupus erythematosus. Int J Dermatol. 1991; 30:635–639.
3. Katayama I, Yamamoto T, Otoyama K, Matsunaga T, Nishioka K. Clinical and immunological analysis of annular erythema associated with Sjögren syndrome. Dermatology. 1994; 189:Suppl 1. 14–17.

4. Katayama I, Kotobuki Y, Kiyohara E, Murota H. Annular erythema associated with Sjögren's syndrome: review of the literature on the management and clinical analysis of skin lesions. Mod Rheumatol. 2010; 20:123–129.

5. Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjögren's syndrome: findings in humans and mice. Arthritis Rheum. 2008; 58:734–743.

6. Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjögren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J Immunol. 2008; 181:2898–2906.

7. Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjögren's syndrome immunopathogenesis. Am J Pathol. 2009; 175:1167–1177.

8. Youinou P, Pers JO. Disturbance of cytokine networks in Sjögren's syndrome. Arthritis Res Ther. 2011; 13:227.

9. Szodoray P, Papp G, Horvath IF, Barath S, Sipka S, Nakken B, et al. Cells with regulatory function of the innate and adaptive immune system in primary Sjögren's syndrome. Clin Exp Immunol. 2009; 157:343–349.

10. Katayama I, Kohno Y, Akiyama K, Ikezawa Z, Kondo N, Tamaki K, et al. Japanese Society of Allergology. Japanese guideline for atopic dermatitis. Allergol Int. 2011; 60:205–220.

11. Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K. Revised Japanese criteria for Sjögren's syndrome (1999): availability and validity. Mod Rheumatol. 2004; 14:425–434.

12. Watanabe T, Tsuchida T. Classification of lupus erythematosus based upon cutaneous manifestations. Dermatological, systemic and laboratory findings in 191 patients. Dermatology. 1995; 190:277–283.

13. Katayama I, Asai T, Nishioka K, Nishiyama S. Annular erythema associated with primary Sjögren syndrome: analysis of T cell subsets in cutaneous infiltrates. J Am Acad Dermatol. 1989; 21:1218–1221.

14. Yamamoto T, Katayama I, Nishioka K. Analysis of T cell receptor Vbeta repertoires of annular erythema associated with Sjögren's syndrome. Eur J Dermatol. 1998; 8:248–251.
15. Arakaki R, Ishimaru N, Saito I, Kobayashi M, Yasui N, Sumida T, et al. Development of autoimmune exocrinopathy resembling Sjögren's syndrome in adoptively transferred mice with autoreactive CD4+ T cells. Arthritis Rheum. 2003; 48:3603–3609.

16. Shiari R, Kobayashi I, Toita N, Hatano N, Kawamura N, Okano M, et al. Epitope mapping of anti-alpha-fodrin autoantibody in juvenile Sjögren's syndrome: difference in major epitopes between primary and secondary cases. J Rheumatol. 2006; 33:1395–1400.
17. Sumida T, Tsuboi H, Iizuka M, Nakamura Y, Matsumoto I. Functional role of M3 muscarinic acetylcholine receptor (M3R) reactive T cells and anti-M3R autoantibodies in patients with Sjögren's syndrome. Autoimmun Rev. 2010; 9:615–617.

18. Miyagawa S, Dohi K, Shima H, Shirai T. HLA antigens in anti-Ro(SS-A)-positive patients with recurrent annular erythema. J Am Acad Dermatol. 1993; 28:185–188.

19. Lowes MA, Kikuchi T, Fuentes-Duculan J, Cardinale I, Zaba LC, Haider AS, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008; 128:1207–1211.

20. Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008; 118:597–607.

21. Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008; 128:2625–2630.

22. McCauliffe DP, Faircloth E, Wang L, Hashimoto T, Hoshino Y, Nishikawa T. Similar Ro/SS-A autoantibody epitope and titer responses in annular erythema of Sjögren's syndrome and subacute cutaneous lupus erythematosus. Arch Dermatol. 1996; 132:528–531.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download