INTRODUCTION
Axillary bromhidrosis is a condition in which body odor is induced by the interaction between apocrine gland secretions and bacteria. Topical treatment, microwave therapy, laser therapy, ultrasonic and/or liposuction curettage and surgical intervention are the current therapeutic options for axillary bromhidrosis. The surgical option is the most effective method. But, it has a high risk of complications including hematoma and necrosis.
New nonsurgical methods may reduce the burden on surgery and the risks for the patient. Laser surgery for axillary bromhidrosis or hyperhidrosis using a CO2 laser and a 1,064 nm Nd:YAG laser have been reported1,2. However, these types of lasers do not specifically target apocrine glands in the dermal fat layers. Therefore, while treatment with these lasers reduce the occurrence of complications, patients are more likely to suffer from recurrence of malodor. In this study, we used a 1,444 nm Nd:YAG laser that has primarily been used for facial lipoplasty and laser liposuction procedures. Removing apocrine glands only within the hypodermic fatty layers using this fiber type of laser has been reported3. Furthermore, the lipolytic effect of the 1,444 nm Nd:YAG laser is superior to the 1,064 nm Nd:YAG laser4. Herein, we report a prospective, follow-up study of the use of a 1,444 nm Nd:YAG laser to treat axillary bromhidrosis.
MATERIALS AND METHODS
Eighteen patients (seven men and 11 women, 36 axillae) with axillary bromhidrosis (five patients also had hyperhidrosis), aged between 18 and 52 years (mean, 33.2 years; range, 18~52 years) were treated with a 1,444 nm Nd: YAG laser (Accusculpt; Lutronic Corp., Goyang, Korea) at the Department of Dermatology, Korea University Ansan Hospital (Ansan, Korea). After irradiation, we followed-up all patients on post-operative days 1, 7, 30, 60, 120, and 180 (median follow-up, 11.9 months). Patients were recruited after Institutional Review Board approval (Korea University Ansan Hospital; IRB No. AS09057-001). Bromhidrosis was diagnosed when the patient and two dermatologists were aware of malodor.
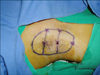
All procedures were performed under local anesthesia. Both operative fields were anesthetized via the infiltration pathway using 20 to 30 ml of 0.5% lidocaine mixed with a 1:100,000 dilution of epinephrine for each field. An approximately 10×5 cm sized laser irradiation area was marked on both axillae. Five to nine small punctures were made with an 18-gauge needle at one side of the affected area (Fig. 1). A cannula was inserted into the subcutaneous and dermal layers throughout the puncture site. Apocrine glands in the subcutaneous and dermal layers were destroyed by irradiation with a fiber type laser at pulse energy of 175 mJ and a pulse rate of 40 Hz (power 7 W). Total energy ranged from 1,706 to 2,267 J. The position and depth of the cannula tip were controlled by transcutaneous guidance with a red helium-neon light. To prevent irreversible skin damage by heat production after tissue-laser interaction, we irradiated the laser in single time per 0.5 to 1.0 cm2 area within 1.5 seconds.
We confirmed destruction of the apocrine glands in all patients through histopathologic examination of a punch biopsy of the treated areas, which was performed on post-operative days 1 and 180. On follow-up days 30, 60, 120, and 180, we measured the severity of remaining odor, postoperative pain, degree of reduced mobility, and the probability of another procedure being required, as well as overall satisfaction. The severity of the remaining malodor was categorized: good (the patient and the two dermatologists positioned within 0.5 m were unaware of malodor), fair (much reduced but occasionally noticeable to the patient and two dermatologists when sweating), and poor (both patient and dermatologists were aware of malodor, or no change compared to the previous malodor)5,6,7. Patient satisfaction was classified as totally satisfied, partially satisfied, or regretful6. Pain and limitation of mobility measurements were recorded on a 10-point scale, with lower scores indicating less severity.
RESULTS
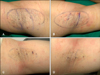
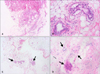
Eighteen patients were followed-up for 6 months postoperatively. Before the laser treatment, 0 axillae were good, six axillae were fair (17%), and 30 axillae were poor (83%). After 1 month, malodor elimination was good in 22 axillae (61%), fair in 12 axillae (33%), and poor in two axillae (6%). After 2 months, malodor elimination was good in 20 axillae (56%), fair in 10 axillae (28%), and poor in six axillae (16%). After 4 months, malodor elimination was good in 20 axillae (56%), fair in 10 axillae (28%), and poor in six axillae (16%). After 6 months, malodor elimination was good in 20 axillae (56%), fair in 12 axillae (33%), and poor in four axillae (11%) (Fig. 2). After laser treatment for 1 month, patient satisfaction was significant in 15 patients (83%), partial satisfaction was observed in three patients (17%), and regretful in no patient (0%). After 2 months, patient satisfaction was significant in 14 patients (78%), partial satisfaction was observed in three patients (17%), and regretful in one patient (5%). After 4 months, patient satisfaction was significant in 13 patients (73%), partial satisfaction was observed in four patients (22%), and regretful in one patient (5%). After 6 months, patient satisfaction was significant in 14 patients (78%), partial satisfaction was observed in three patients (17%), and regretful in one patient (5%) (Fig. 3). Pain and limitation of mobility were significantly reduced by 1 week postoperatively, and were almost resolved within 4 weeks postoperatively. Adverse effects were mild compared with manual surgery. Of the 36 axillae treated, 20 (56%) presented with ecchymosis, which usually resolved within a few weeks (Fig. 4). Furthermore, superficial epidermis necrosis was evident in four axillae (11%) (which healed through conservative management), hypertrophic scarring in two axillae (which were almost flattened through intralesional injection of 8 mg/ml 6% triamcinolone), and temporary skin pigmentation in six axillae (which almost cleared spontaneously within 1 year; 17%). We did not observe any complications such as hematoma, seroma, granuloma, wound infection, or dehiscence. A histopathological examination revealed decreased density and significant alterations to the apocrine glands after treatment (Fig. 5).
DISCUSSION
Surgical intervention for axillary bromhidrosis can lead to a higher therapeutic completion rate and a lower rate of condition recurrence but may result in complications such as axillary nerve plexus damage, postoperative pain, hemorrhage, edema, hematoma, and severe limitation of mobility post-operatively8. In addition, the outcome of surgery differs among surgeons. For these reasons, no consensus has been reached on the appropriate surgical method for treating axillary bromhidrosis. Many patients prefer to be treated for bromhidrosis by minimal invasive therapies; the many different available procedures include laser therapies that dissolve fat and destroy the apocrine glands. Microwave device therapy, which isolates and heats target tissue, was introduced recently9,10. However, safety and efficacy data are lacking.
While both 1,064 nm and 1,320 nm Q-switched Nd:YAG lasers (Smartlipo, Cynosure Inc., Westford, MA, USA; Coollipo, CoolTouch Inc., Roseville, CA, USA; Cool-Touch, CoolTouch Inc.) are currently used to dissolve fat3, these two wavelengths are less specific to fat cells. The 1,444 nm type laser more markedly absorbs in fat than water compared to other wavelengths. A greater lipolytic effect of the 1,444 nm wavelength Nd:YAG laser compared to the 1,064 nm Nd:YAG laser has been reported. That same study also reported that as epidermal temperature increases with 1,444 nm beam therapy, the procedure provides identical energy at the same depth in the hypodermic fat layer as is achieved using other wavelengths4.
We hypothesized that apocrine glands in fatty tissues would be more specifically destroyed by the 1,444 nm laser through a subdermal interstitial approach. At 180 days of follow-up, malodor elimination was good in 20 axillae (56%), fair in 12 axillae (33%), and poor in four axillae (11%). Most patients (95%) were satisfied with their results in terms of effectiveness and side-effects. Although some differences between the current and prior bromhidrosis treatment studies were evident, there was no significant difference in the clinical outcomes between the 1,444 nm laser and other methods5,6,11. In a study that involved subcutaneous treatment using a 1,064 nm laser, malodor elimination was good in four patients (33.3%), fair in eight patients (66.7%), and poor in no patients5. Surgical treatment resulted in malodor elimination that was good in 398 axillae (95%), fair in 14 axillae (3.4%), and poor in no axillae (0%)6. In a study involving liposuction treatment, malodor elimination was very good in 13 patients (11.4%), good in 79 patients (69.2%), and poor in 22 patients (19.3%).
After irradiation, we followed-up all patients on postoperative days 1, 7, 30, 60, 120, and 180, and observed superficial epidermal necrosis in four axillae (11%), which healed during conservative management. Epidermal necrosis may have been the result of technical error, such as laser tip injury and incorrect target treatment. Hypertrophic scarring occurred in two axillae (6%) and temporary skin pigmentation in six axillae (17%). Compared with other methods, including surgical excision or liposuction, the 1,444 nm laser treatment had less significant side-effects, such as hematoma and wound infection6,11.
We compared the post-treatment side effects, recurrence, and patient satisfaction between laser-therapy and surgical intervention, and noted no significant differences in the clinical outcome1,2. With exceptions of heat-induced skin crust and mild ecchymosis, laser-therapy did not induce any long-lasting side effects. However, over-treatment or over-coagulation may potentially increase the risk of skin necrosis and other injuries. Therefore, laser operators should assess any superficial skin color changes and measure superficial temperature in real-time.
Comparison of the sustained benefits of laser-treatment and surgical intervention was unclear. However, acute phase complications such as postoperative pain and restricted movement were less evident in the laser-therapy patients. For this reason, we recommend the therapeutic process outlined in this report as a possible treatment of choice for bromhidrosis. However, we continue to recommend that the patient to choose the surgical method for total removal of malodor with a low likelihood of condition recurrence.
In conclusion, subdermal interstitial coagulation treatment with a 1,444 nm Nd:YAG laser may be a less invasive and effective alternative therapy for axillary bromhidrosis. We are currently performing a prospective clinical study with a larger cohort to investigate the ideal laser configuration and to define systematic and standardized protocols.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download