Abstract
Background
Acquired perforating dermatosis (APD) is histopathologically characterized by transepidermal elimination of materials from the upper dermis. APD can be divided into four diseases: Kyrle's disease, perforating folliculitis, elastosis perforans serpiginosa, and reactive perforating collagenosis. APD is usually associated with systemic diseases, especially diabetes mellitus or chronic renal failure. So far, there have only been a few Korean studies of APD, which have a limited number of patients.
Objective
The aim of this study is to evaluate the clinical and histopathologic characteristics of 30 cases of APD and to examine the association with systemic diseases.
Methods
We retrospectively reviewed the medical records and biopsy specimens of 30 patients who were diagnosed with APD.
Results
The mean age was 55.5 years, and the average duration of the lesion was 7.8 months. The lower extremities (73.3%) were the most frequently occurring sites of the lesion. Twenty-five patients (83.3%) had pruritus, and Koebner's phenomenon was present in 11 patients. Patients of 63.3% had at least one systemic disease. Diabetes mellitus (n=17, 56.7%) and chronic renal failure (n=10, 33.3%) were the most commonly associated conditions. Most patients received topical steroids (93.3%) and antihistamines (80.0%). The most common histopathologic type was reactive perforating collagenosis (n=23, 73.3%).
Conclusion
In this study, most patients had a systemic association to the diseases. Therefore, we suggest that further evaluation is necessary for patients who present with APD. This includes reviewing patient's comprehensive past medical history, clinical exam, and additional diagnostic testing to check for the possibility of associated systemic diseases.
Acquired perforating dermatosis (APD) is histopathologically characterized by transepidermal elimination of various dermal substances. APD can be divided into four diseases, according to the types of epidermal disruption and the natures of the eliminated materials: Kyrle's disease (KD), perforating folliculitis (PF), elastosis perforans serpiginosa (EPS), and reactive perforating collagenosis (RPC)1. APD is usually associated with diabetes mellitus (DM)2 or chronic renal failure (CRF)3. In recent years, it has also been reported to be associated with other systemic diseases, such as non-diabetic hemodialysis4, hepatic5 and endocrinologic disorders5, hepatocellular carcinoma6, Hodgkins' lymphoma7, acute leukemia8, acquired immune deficiency syndrome (AIDS)9, tuberculosis10, pulmonary aspergillosis11, neurodermatitis5, atopic dermatitis12, scabies13, and pregnancy14.
To date, there have been only a few studies of APD, including case reports in Korean literature, which have a limited number of patients and make it difficult for a literature review. We report the clinical and histopathological features of 30 cases with APD and clarify the association with pathogenesis of APD and comorbidities. To our knowledge, this is the first report of its kind and the largest study on the clinicopathological features of APD, including all classical types of APD performed in Korea.
We performed a retrospective review of the medical records and biopsy specimens of 30 patients who had been histopathologically diagnosed with APD between January 1997 and October 2012 in the Departments of Dermatology at the Eulji General Hospital and Chung-Ang University Hospital.
The following clinical data was collected: age, gender, duration, distribution of skin lesions, clinical configuration, associated symptoms, presence of Koebner's phenomenon, associated systemic diseases, and treatment.
All of the cutaneous samples were fixed in formalin, and processed and embedded in paraffin. The sections were stained with hematoxylin and eosin. If paraffin blocks were available, new sections were obtained for histochemical staining for all cases; Verhoeff-van Gieson and Masson trichrome stains were used to evaluate the elimination of elastic or collagen fibers. The samples were classified as KD, PF, EPS and RPC, according to the pathologic features and eliminated materials.
Among the 30 patients, 12 were males and 18 were females. Patients ranged from 30 to 83 years of age. The mean age was 55.5 years. The highest frequency of APD was between 50 and 59 years of age (26.7%).
Duration of lesions varied from 1 month to 5 years with the average of 7.8 months. The distribution of the lesions was diffuse and variable. The lower extremities (73.3%) were the most commonly involved site, followed by the trunk (60.0%), and the upper extremities (40.0%). Multi-site involvement was observed in 14 patients (46.7%). There was one case of APD occurring on the face and scalp. However, no APD was observed on the palms and soles. Twenty-five patients (83.3%) had pruritus, and 2 (6.7%) had pain.
Based on the clinical configuration of the lesion, we divided patients into the following four groups: KD-like hyperkeratotic papules, PF-like follicular infiltrating papules, EPS-like serpiginous hyperkeratotic plaques, and RPC-like keratotic plugged, umbilicated papules. The most common configuration was RPC-like lesions (n=21, 66.7%). Koebner's phenomenon was observed in 11 patients (Fig. 1).
Nineteen (63.3%) patients had at least one systemic disease. DM (n=17, 56.7%) was the most commonly associated condition, followed by CRF (n=10, 33.3%), hypertension (n=6, 20.0%), hepatitis (n=2, 6.7%), hypothyroidism (n=1, 3.3%), pregnancy (n=1, 3.3%), and chronic obstructive pulmonary disease (n=1, 3.3%). Of the 17 patients with DM, 2 (11.8%) had insulin-dependent DM (IDDM), and 15 (88.2%) had non-insulin-dependent DM (NIDDM). Among the patients with CRF, 5 (50%) had been treated on dialysis (3 on hemodialysis and 2 on peritoneal dialysis). Eleven patients (36.7%) had no other systemic diseases.
Nine patients had other dermatologic disorders; 5 (16.7%) of which had xerotic eczema, 3 (10.0%) had atopic dermatitis, and 1 (3.3%) had stasis dermatitis. One patient was a pregnant woman at 29 weeks gestation.
Twenty-eight patients (93.3%) received topical steroids, and 24 (80.0%) received antihistamines. An intralesional triamcinolone injection was administered to 11 patients (33.3%), narrow-band ultraviolet B (NB-UVB) to 7 patients (23.3%), systemic retinoid therapy to 3 patients, and cryotherapy to 1 patient (3.3%). Almost all patients improved after treatment, but 1 patient (3.3%) had no response to treatment.
Histopathological features of 23 cases (73.3%) showed cup-shaped invagination of the epidermis plugged with necrotic inflammatory debris. There were vertically oriented collagen bundles at the base of the lesions. Massontrichrome staining showed transepidermal elimination of the collagen bundles. The overall histological features were consistent with RPC (Fig. 2). Histopathological features of 5 cases (16.7%) showed a parakeratotic plug containing basophilic debris within a central epidermal invagination, consistent with KD (Fig. 3). In addition, histopathological features of 1 case (3.3%) showed narrow transepidermal channels containing coarse elastic fibers and basophilic debris. Verhoeff-van Gieson staining revealed transepidermal elimination of elastic fibers, consistent with EPS (Fig. 4). One case (3.3%) revealed a dilated follicular infundibulum filled with mixture of keratin, basophilic debris, inflammatory cells, and degenerated collagen fibers-consistent with PF (Fig. 5).
Perforating disorders are rare conditions, characterized by transepidermal elimination of dermal substances. In 1989, Rapini et al.15 reported several cases of perforating dermatosis in patients with DM or renal disease. They noted that histopathological findings were no difference from the previously defined perforating disorders. They suggested that this process be referred to as 'acquired perforating dermatosis.' Since then, APD has been considered to be an entity that represents perforating disorders, occurring in patients with diabetes or chronic renal failure. However, many literatures still uses other terms such as acquired perforating dermatosis, acquired perforating disorders, and perforating dermatoses.
APD is most often found in middle-aged adults. The lesions are most often developed on the extensor surfaces of the extremities. However, any site on the cutaneous surface can be affected, including the trunk and scalp. There has been a report of KD on the conjunctiva and buccal mucosa16. In the current study, the lower extremities were the most frequently involved sites, which were consistent with findings from previous reports17,18,19.
Pruritus was the most frequent symptom in patients with APD17. In our study, pruritus was presented in the most patients (83.3%). Two patients were presented with pain, which was a rare symptom for APD. Similarly, Saray et al.17 reported 22 cases with APD, 2 of which presented with pain. In APD patients, Koebner's phenomenon may occur after scratching, rubbing or trauma to the lesion18. Koebner's phenomenon was observed in 11 patients from our study.
The pathogenesis of APD remains unknown. Trauma from scratching, as a major trigger of perforating disorders, may induce damage of the epidermis or dermal collagen, leading to transepidermal elimination of collagen or elastic fibers20. This hypothesis is supported by the predilection of the lesions to the previous areas of trauma, and the presence of Koebner's phenomenon17. Another hypothesis is the alteration in collagen or elastic fibers due to metabolic disturbances or microdeposition of substances, such as calcium salts21. Underlying dermal microvasculopathy related to DM is another suggested predisposing factor of APD. Diabetic vasculopathy induces a hypoxic state, in which the trauma from scratching causes dermal necrosis. This hypothesis has been supported by the findings of a positive stain with periodic acid-Schiff, and thickening of the vessel walls in the upper dermis of diabetic patients with APD18.
APD is classified into 4 diseases, according to the type of epidermal disruption and the nature of the eliminated material: KD, PF, EPS and RPC1. Classically, KD, PF and RPC present similar clinical features, characterized by umbilicated papules with central white, keratotic crusts. However, EPS presents with hyperkeratotic papules in a serpiginous distribution22. In our study, we observed one case that presented with serpiginous hyperkeratotic plaques, which was EPS confirmed by histopathology. Histopathologically in RPC, collagen bundles perforate through the epidermis. In EPS, elastic fibers are eliminated through the epidermis. In PF, degenerated follicular units with or without collagen and elastic fibers are eliminated, and in KD, amorphous dermal materials without collagen or elastic fibers perforate through the epidermis17. We classified the 30 cases of APD into the above-mentioned 4 diseases according to the histopathologic features, and observed all classic types of perforating dermatosis. The RPC (n=23) was the most common type, followed by KD (n=4), EPS (n=1) and PF (n=1). Rapini et al.15 reported combined transepidermal elimination of both collagen and elastic fibers in four patients with APD. They proposed that varying histological findings in APD may represent the different stages or different types of lesions in the same pathological process. However, Saray et al.17 suggested that APD represents the broad spectrum of perforating disorders rather than the variants of the same pathological process. We also could not observe the overlapping histologic features in the same lesion. Therefore, our study findings support a suggestion that APD is the spectrum of perforating disorders.
APD is usually associated with systemic diseases, especially DM, CRF or both. In our patients, DM was also the most commonly associated disease (56.7%). Two of our patients had IDDM, and 15 had NIDDM. A lot of studies have debated which type of DM is more strongly associated with APD. Similar to our result, some authors found that NIDDM is more frequent in patients with APD5,23. However, Morton et al.21 reported that APD is more often in IDDM, compared with NIDDM. Consequently, Hong et al.18 suggested that the DM type does not appear to be a predisposing factor in APD.
In majority (90%) of our patients with CRF, the underlying cause of CRF was DM. Morton et al.21 reported that DM is the most frequent cause of CRF in APD patients. However, some authors have reported cases of renal impairment, not due to diabetes, including obstructive nephropathy, hypertensive nephrosclerosis, acquired immune deficiency syndrome, chronic nephritis, and anuria4,5,9,24,25. In our study, we also found one patient with non-diabetic CRF. Thus, based on the present findings, as well as the previous reports, CRF itself might be an important predisposing factor in development of APD. In patients with CRF, APD often developed, following dialysis, but also occurred before dialysis5. Among our patients with CRF, 5 patients had undergone either hemodialysis or peritoneal dialysis. In all of these patients, the lesions had developed after the initiation of dialysis.
In recent years, there have been many reported cases of APD associated with other various diseases, such as malignancy, hepatic and endocrinologic disorders, AIDS, tuberculosis, pulmonary aspergillosis, atopic dermatitis, scabies, and pregnancy17,19. In the current study, congestive heart failure, hypothyroidism, pregnancy, hypertension, hepatitis, chronic obstructive pulmonary disease, xerotic eczema, atopic dermatitis, and stasis dermatitis were observed in APD patients. However, these associated diseases, except pregnancy, were accompanied with DM, CRF, or both. Therefore, it could not be explained that these diseases contribute to the occurrence of APD by itself.
We observed a case with APD occurring in pregnancy, which was an unusual associated condition. Healy et al.14 described a case with APD occurring in pregnancy. She had undergone polymorphic eruption of pregnancy before the development of APD. Thus, they proposed that persistent scratching might be a contributing factor. However, our patients had no other previous cutaneous problems and had no dermatologic diseases during pregnancy, such as pruritic urticarial papules and plaques in pregnancy. It remains unknown why APD develops in pregnant woman without an underlying disease.
In this study, there were 9 patients with other dermatologic diseases, such as xerotic eczema, atopic dermatitis and stasis dermatitis. Although all of these patients had underlying DM or CRF, we suggested that associated dermatologic problems might contribute to development of APD because those associated dermatologic diseases commonly presented with itching and scratching.
Treatment of APD can be difficult and various therapies have been reported. The most commonly described are topical and intralesional steroids, oral antihistamines and topical retinoids. Others include NB-UVB, psoralen plus ultraviolet A, oral retinoid, allopurinol, doxycycline and methotrexate14,19. Most of our patients were treated with topical steroids, oral antihistamine and intralesional triamcinolone injection. The key point of treatment in APD is relief of pruritus. If the patient is protected then it is probable that APD has improved.
In the current study, RPC is the most common observed type of APD, followed by KD, EPS and PF. The results of the current study were consistent with previous studies of APD performed in other countries, including age, duration, distribution of skin lesion, clinical configuration, associated symptom, presence of Koebner's phenomenon, and associated systemic diseases. In this study, most patients had systemic association to the diseases. Therefore, we suggest that further evaluation including comprehensive past medical history, clinical exam and additional diagnostic testing for possibility of associated systemic diseases is necessary for patients who present with APD. On the other hand, the interesting fact was that there were 11 patients who had no other systemic diseases in our study. For those reasons, we propose further studies to identify the mechanisms of development of APD in healthy individuals. Additionally it is necessary to certify the mechanism of itching in APD patients.
Figures and Tables
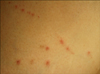 | Fig. 1Multiple erythematous linear arranged hyperkeratotic umbilicated papules on the trunk of patient 19. Note Koebner phenomenon from scratching. |
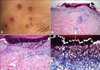 | Fig. 2(A) Reactive perforating collagenosis-like keratotic plugged, umbilicated papules on the lower extremities of patient 14. (B) Cup-shaped invagination of the epidermis plugged with necrotic inflammatory debris (H&E, ×40). (C) There were vertically oriented collagen bundles at the base of the lesions (H&E, ×100). (D) Transepidermal elimination of the collagen bundles (Masson-trichrome stain, ×200). |
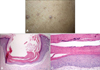 | Fig. 3(A) Kyrle's disease-like hyperkeratotic papules on the lower extremities of patient 3. (B) Central epidermal invagination containing parakeratotic debris (H&E, ×40). (C) Parakeratotic plug containing basophilic debris (H&E, ×200). |
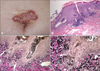 | Fig. 4(A) Elastosis perforans serpiginosa-like serpiginous hyperkeratotic plaques on the abdomen of patient 8. (B) Narrow transepidermal channels containing coarse elastic fibers and basophilic debris (H&E, ×40). (C, D) Transepidermal elimination of elastic fibers (Verhoeff-van Gieson stain; C: ×100, D: ×200). |
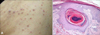 | Fig. 5(A) Perforating folliculitis-like follicular infiltrating papules on the thigh of patient 25. (B) Dilated follicular infundibulum filled with mixture of keratin, basophilic debris, inflammatory cells and degenerated collagen fibers (H&E, ×100). |
Table 1
Clinical features of 30 patients

APD: acquired perforating dermatosis, M: male, F: female, LEx: lower extremities, UEx: upper extremities, NIDDM: non-insulin-dependent diabetes mellitus, CRF: chronic renal failure, CHF: congestive heart failure, HD: hemodialysis, HTN: hypertension, IDDM: insulin-dependent diabetes mellitus, PD: peritoneal dialysis, COPD: chronic obstructive pulmonary disease, NB-UVB: narrow-band ultraviolet B, TA ILI: intralesional triamcinolone injection.
References
1. Miller MK, Friedman RJ, Naik NS, Heilman ER, Nousari CH. Degenerative diseases and perforating disorders. In : Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Lever's histopathology of the skin. 10th ed. Philadelphia: Lippincott Williams & Wilkins;2009. p. 391–396.
2. Poliak SC, Lebwohl MG, Parris A, Prioleau PG. Reactive perforating collagenosis associated with diabetes mellitus. N Engl J Med. 1982; 306:81–84.

3. Cochran RJ, Tucker SB, Wilkin JK. Reactive perforating collagenosis of diabetes mellitus and renal failure. Cutis. 1983; 31:55–58.
4. Iyoda M, Hayashi F, Kuroki A, Shibata T, Kitazawa K, Sugisaki T, et al. Acquired reactive perforating collagenosis in a nondiabetic hemodialysis patient: successful treatment with allopurinol. Am J Kidney Dis. 2003; 42:E11–E13.

5. Faver IR, Daoud MS, Su WP. Acquired reactive perforating collagenosis. Report of six cases and review of the literature. J Am Acad Dermatol. 1994; 30:575–580.
6. Kiliç A, Gönül M, Cakmak SK, Gül U, Demiriz M. Acquired reactive perforating collagenosis as a presenting sign of hepatocellular carcinoma. Eur J Dermatol. 2006; 16:447.
7. Eigentler TK, Metzler G, Brossart P, Fierlbeck G. Acquired perforating collagenosis in Hodgkin's disease. J Am Acad Dermatol. 2005; 52:922.

8. Karpouzis A, Tsatalas C, Sivridis E, Kotsianidis I, Margaritis D, Kouskoukis C, et al. Acquired reactive perforating collagenosis associated with myelodysplastic syndrome evolving to acute myelogenous leukaemia. Australas J Dermatol. 2004; 45:78–79.

9. Bank DE, Cohen PR, Kohn SR. Reactive perforating collagenosis in a setting of double disaster: acquired immunodeficiency syndrome and end-stage renal disease. J Am Acad Dermatol. 1989; 21:371–374.

10. Zelger B, Hintner H, Auböck J, Fritsch PO. Acquired perforating dermatosis. Transepidermal elimination of DNA material and possible role of leukocytes in pathogenesis. Arch Dermatol. 1991; 127:695–700.

11. Kim JH, Kang WH. Acquired reactive perforating collagenosis in a diabetic patient with pulmonary aspergillosis. Cutis. 2000; 66:425–430.
12. Thiele-Ochel S, Schneider LA, Reinhold K, Hunzelmann N, Krieg T, Scharffetter-Kochanek K. Acquired perforating collagenosis: is it due to damage by scratching? Br J Dermatol. 2001; 145:173–174.

13. Hinrichs W, Breuckmann F, Altmeyer P, Kreuter A. Acquired perforating dermatosis: a report on 4 cases associated with scabies infection. J Am Acad Dermatol. 2004; 51:665–667.

14. Healy R, Cerio R, Hollingsworth A, Bewley A. Acquired perforating dermatosis associated with pregnancy. Clin Exp Dermatol. 2010; 35:621–623.

15. Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. Evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989; 125:1074–1078.

16. Alyahya GA, Heegaard S, Prause JU. Ocular changes in a case of Kyrle's disease. 20-year follow-up. Acta Ophthalmol Scand. 2000; 78:585–558.

17. Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol. 2006; 20:679–688.

18. Hong SB, Park JH, Ihm CG, Kim NI. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004; 19:283–288.

19. Satti MB, Aref AH, Raddadi AA, Al-Ghamdi FA. Acquired reactive perforating collagenosis: a clinicopathologic study of 15 cases from Saudi Arabia. J Eur Acad Dermatol Venereol. 2010; 24:223–227.

20. Mehregan AH, Schwartz OD, Livingood CS. Reactive perforating collagenosis. Arch Dermatol. 1967; 96:277–282.

21. Morton CA, Henderson IS, Jones MC, Lowe JG. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol. 1996; 135:671–677.

22. Minocha JS, Schlosser BJ. Acquired perforating disorders. In : Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. Fitzpatrick's dermatology in general medicine. 8th ed. New York: McGraw-Hill Medical;2012. p. 727–773.
23. Cohen RW, Auerbach R. Acquired reactive perforating collagenosis. J Am Acad Dermatol. 1989; 20:287–289.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download