Abstract
Background
Atopic dermatitis (AD) patients suffer from xerosis. Proper skin care, including the use of emollients, may help improve xerosis and minimize disease exacerbation. Lactobacillus sakei probio 65, isolated from the Korean vegetable-based product kimchi, can decrease interleukin 4 and immunoglobulin E levels and inhibit Staphylococcus aureus. Moreover, it has reportedly shown positive dermatological effects in both animal and clinical studies.
Objective
To compare the effects of an emollient that contains Lactobacillus (treated) with a normal emollient (control) on AD.
Methods
This double-blind, randomized, split-body clinical trial involved 28 patients with AD. The patients applied the Lactobacillus-containing emollient on one side of their body and the control emollient on the other side twice daily for 4 weeks. Trans-epidermal water loss (TEWL) and skin capacitance were evaluated and investigator global assessment and the visual analogue scale (VAS) were administered on weeks 0, 1, 2, and 4.
Atopic dermatitis (AD) is generally characterized by pruritic skin lesions and chronic eczematous lesions. It may occur in people of any age, but disease onset is more common in preschoolers1. The management of AD requires a multipronged approach that includes repair of barrier function and general skin care, use of topical or systemic agents, and the identification and elimination of precipitating or exacerbating factors2. Hydration of the skin is particularly important because dry skin gives rise to microfissures and cracks that allow the entry of pathogens, antigens, and irritants, thereby exacerbating the disease3.
The regular use of emollients has many beneficial effects in AD. It improves the appearance and symptoms of dry skin3 and may reduce the need for topical corticosteroids by approximately 50%4. A study found that emollients enhance the response to treatment with topical corticosteroids5. In addition, a pilot study showed that emollients can prevent the development of AD in high-risk infants6. Indeed, some patients only use emollients due to concerns about the side effects of topical corticosteroids7.
Several studies suggest that incorporating Lactobacillus may improve the performance of emollients in AD. In an animal AD model study, topical application of medicinal herbs fermented with Lactobacillus plantarum seemed to have a better therapeutic effect than the control and oral administration groups8. In addition, Segawa et al.9 reported that intrarectal administration of Lactobacillus-derived polyphosphate enhances the epithelial barrier function in mouse small intestine.
L. sakei probio 65, the stain used in this study, was isolated from kimchi, which is a traditional fermented Korean food that is believed to reduce the risk of chronic diseases. A range of bacteria are involved in the kimchi fermentation process, with Lactobacillus being one of the major species. Of the various strains of this bacterium, L. sakei probio 65, exhibited the most potent inhibitory activity against Staphylococcus aureus growth in preliminary experiments. It also induced immunological and clinical improvements in an animal study and a clinical study10,11. The present clinical trial was performed to determine whether the efficacy of emollients in AD could be improved by incorporating L. sakei.
In the present study, L. sakei probio 65-containing emollient was tested for safety as a topical medication in a rabbit model, after which patients with AD were recruited and asked to apply the L. sakei probio 65-containing emollient to one randomly selected side of the body and a normal emollient (control) to the other side. The clinical outcome and electrical skin barrier functions of each side were then evaluated. The control and L. sakei probio 65-containing emollients were identical except for the presence of L. sakei probio 65 in the latter.
The rabbits were divided into three groups. The control group received normal saline, Group 1 received L. sakei probio 65 extract, and Group 2 received an L. sakei probio 65-containing emollient. This study was approved by the Chungnam National University Institutional Animal Care and Use Committee of Korea (CNUCOM-2007-017).
The clinical trial was performed between the end of June and the beginning of October 2011. The subjects were followed-up until November 2011. All subjects were recruited from the Dermatology Clinics of Chungnam National University Hospital in Daejeon, Korea. The patients or parents/guardians were provided with written informed consent before study entry. This study was approved by the Chungnam National University Hospital Institutional Review Board (IRB no. 1106-40). The 30 patients who participated in the study had been diagnosed with bilateral AD for at least 6 months according to Hanifin's criteria12. The mean age of the patients was 14.2 years (range 3~37 years), and there were 20 males and 10 females. The AD (SCORing atopic dermatitis, SCORAD) total score of the patients ranged from 13.5 to 50.3 (mean 30.4). Patients who were treated with cyclosporine or systemic corticosteroids 4 weeks before the study were excluded. Continuing use of antihistamines and topical steroids was permitted. At baseline visit, one side of the body of each patient was randomly assigned to receive the L. sakei probio 65-containing emollient, while the other side was treated with the control emollient. The emollients were both applied twice daily.
Clinical visits were scheduled at baseline and after weeks 1, 2, and 4. The clinical outcome was evaluated by using clinical and objective parameters. Clinical parameters were assessed using the investigator's global assessment (IGA) and the visual analogue scale (VAS) for pruritus. The IGA of both sides of the body was graded by a dermatologist on a five-point scale (0, clear; 1, nearly clear; 2, mild; 3, moderate; 4, severe). The VAS assessments were performed by the patients themselves. Each patient was asked to score the severity of their pruritus subjectively on a scale of 0 to 10. Objective parameters included trans-epidermal water loss (TEWL) and skin capacitance. The TEWL of both sides was measured by using a Tewameter TM210 (CK electronic, Cologne, Germany). The skin capacitance was assessed using a Corneometer CM825 (CK electronic). Each objective parameter was measured three times at 23℃ and at a relative humidity of 54% to 56%, after which the average measurement was calculated.
The baseline patient data were analyzed by using Student's t-test. The data of the treated side of the body were compared to those of the control side by using a repeated measure analysis of variance. IBM SPSS Statistics 19.0 software (IBM Co., Armonk, NY, USA) was used for statistical analyses. p-values of <0.05 were considered to be statistically significant.
Fig. 1A and B show the eye stimulation and skin stimulation test results, respectively. None of the three groups showed a response 24, 48, and 72 hours after being exposed to the control or test agent.
Of the 30 subjects who were enrolled, two were lost to follow-up over the 4-week study period.
The baseline clinical data of the subjects are summarized in Table 1. The treatment and control sides did not differ significantly in terms of baseline disease severity. As a reault of random selection of the treatment side, the test emollient was applied to the right antecubital side in 10 subjects, the left antecubital side in 9 patients, the right popliteal side in 4 patients, and the left popliteal side in 5 patients.
Fig. 2 shows digital photographs of cases where substantial improvement was observed after the L. sakei probio 65-containing emollient was applied. Fig. 3A shows the IGA scores during the 4-week study period. None of the treatment sides were worse than their control sides, and in three cases, the treatment side had a better result than the control side. The mean difference between the IGA values of the treatment and control sides increased substantially from -0.04±0.19 at the baseline visit to -0.04±0.43 at week 1, 0.14±0.59 at week 2, and 0.18±0.61 at week 4. However, these differences were not statistically significant (p=0.366).
The VAS scores during the 4 week study period are shown in Fig. 3B. The mean VAS scores of both the treatment and control sides decreased during weeks 1, 2, and 4, and the mean difference between the VAS values of the treatment and control sides increased significantly from -0.04±0.19 at the baseline visit to -0.04±0.43 at week 1, 0.39±0.79 at week 2, and 0.57±0.96 at week 4 (p=0.006). This suggests that the treatment side improved faster than the control side.
The TEWL data during the 4 week study period are shown in Fig. 3C. The mean TEWL of both the treatment and control sides decreased during weeks 1, 2, and 4, and the mean difference between the TEWL values of the treatment and control sides increased significantly from -2.74±4.19 at the baseline visit to -0.51±4.64 at week 1, 3.99±7.05 at week 2, and 5.59±9.75 at week 4 (p=0.007). This suggests that the TEWL of the treated side improved faster than that of the control side.
Skin capacitance during the 4 week study period is shown in Fig. 3D. The mean skin capacitance of both the treatment and control sides increased at weeks 1, 2, and 4, and the mean difference between the skin capacitance values of the treatment and control sides increased significantly from -1.45±4.10 at the baseline visit to -0.89±5.53 at week 1, 1.75±4.31 at week 2, and 5.95±7.13 at week 4 (p=0.001). This suggests that the treatment side developed better skin capacitance than the control side.
This study is the first randomized split-body controlled trial of Lactobacillus extract for the treatment of AD. No irritation was observed from the use of the extract in the animal study. The treated sides of the body showed no significant adverse effects when compared to the control sides in the clinical study. The TEWL, VAS, and skin capacitance values of the treated sides were significantly improved relative to the values of the control sides. However, the IGA values of the treated sides did not differ significantly from those of the control sides. This result can be probably explained by the short study period and the use of only five IGA grades in the scoring. Overall, the results suggest that the L. sakei probio 65-containing emollient is safe and beneficial in patients with AD.
Recently, numerous studies have suggested that the ability of Lactobacillus to inhibit allergic reactions may relate to its ability to improve the Th1/Th2 balance13,14. Moreover, Lactobacillus was shown to inhibit S. aureus growth and to improve the epithelial barrier function9,15,16. However, a study on the ex-vivo immunomodulatory effects of probiotics in children with AD returned inconclusive results17. Most of the studies on Lactobacillus used the L. rhamnosus strain to evaluate the clinical and immunological responses. However, we chose L. sakei probio 65 as it was used in two studies previously. Park et al.10 described an animal study involving the oral administration of L. sakei probio 65 in which the treated group exhibited better clinical improvement and lower interleukin (IL)-4 and immunoglobulin (Ig)-E levels than the control group. In addition, a 12-week, double-blind, placebo-controlled trial showed that oral administration of L. sakei probio 65 resulted in clinical improvement of patients with AD11. In the present study, a L. sakei probio 65-containing emollient was applied topically in patients with AD. Studies on topical Lactobacillus application in patients with AD are rare. Joo et al.8 conducted a study using an AD rat model where medicinal herbs fermented with L. plantarum were administered topically. They found that topical application resulted in better histological results than oral administration. Two other studies of topical application of Lactobacillus in burn victims showed that this treatment improved tissue repair and inhibition of bacteria18,19. Thus, the few studies on the topical use of Lactobacillus support the observations of the present study.
L. sakei probio 65-containing emollients may have some advantages over other emollients in the treatment of patients with AD. First, L. sakei can inhibit the growth of S. aureus, a known immunological trigger1 and cause of common complications (such as impetigo)20. Second, it has immunomodulatory effects that are likely to decrease the IL-4- and Ig-E-induced acute phase of AD17,18, although additional studies are needed to confirm that topical application of such an emollient has these effects as well. Third, Lactobacillus-derived polyphosphate improved the ability of the intestinal epithelium to defend itself from injurious stimulants9,15,16. Thus, Lactobacillus-derived polyphosphate may have similar effects on the skin epithelium. These potential advantages of L. sakei probio 65-containing emollients may explain why topical application of the emollient improved the skin barrier and resulted in the improvement of symptoms in AD patients in the present study.
The limitations of the present study are that it was a single center study involving a relatively small group of subjects who were treated for a short period. Moreover, there was concomitant treatment with topical steroids in most cases. It is unclear whether the use of topical steroids affected the efficacy of the L. sakei probio 65-containing emollient. However, since the patients in the present study used topical steroids on both sides, the observed effects of the L. sakei probio 65-containing emollient remain clinically meaningful. However, the topical steroid-independent effects of L. sakei probio 65 should be evaluated in the future.
In summary, this double-blind, randomized, split-body study, in which many variables were controlled, suggests that L. sakei probio 65-containing emollients may be beneficial and safe for use in patients with AD who are using a topical steroid cream.
Figures and Tables
Fig. 1
Three groups showed no responses in eye stimulation test (top: PBS, middle: Lactobacillus sakei probio 65 extract containing emollient, bottom: L. sakei probio 65 extract) (A) and skin stimulation test (left: PBS, middle: L. sakei probio 65 extract, right: L. sakei probio 65 extract containing emollient) (B). PBS: phosphate buffer saline.
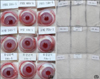
Fig. 2
Effect of emollients containing Lactobacillus in atopic dermatitis patients at basline (A) and after 4 weeks (B).

ACKNOWLEDGMENT
This study was supported by a grant from the Korea Ministry of Health and Welfare (A091121).
References
2. Leung DY, Hanifin JM, Charlesworth EN, Li JT, Bernstein IL, Berger WE, et al. Work Group on Atopic Dermatitis. Disease management of atopic dermatitis: a practice parameter. Joint Task Force on Practice Parameters, representing the American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 1997; 79:197–211.
3. Hanifin JM, Cooper KD, Ho VC, Kang S, Krafchik BR, Margolis DJ, et al. Guidelines of care for atopic dermatitis, developed in accordance with the American Academy of Dermatology (AAD)/American Academy of Dermatology Association "Administrative Regulations for Evidence-Based Clinical Practice Guidelines". J Am Acad Dermatol. 2004; 50:391–404.
4. Lucky AW, Leach AD, Laskarzewski P, Wenck H. Use of an emollient as a steroid-sparing agent in the treatment of mild to moderate atopic dermatitis in children. Pediatr Dermatol. 1997; 14:321–324.

5. Szczepanowska J, Reich A, Szepietowski JC. Emollients improve treatment results with topical corticosteroids in childhood atopic dermatitis: a randomized comparative study. Pediatr Allergy Immunol. 2008; 19:614–618.

6. Simpson EL, Berry TM, Brown PA, Hanifin JM. A pilot study of emollient therapy for the primary prevention of atopic dermatitis. J Am Acad Dermatol. 2010; 63:587–593.

7. Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000; 142:931–936.

8. Joo SS, Won TJ, Nam SY, Kim YB, Lee YC, Park SY, et al. Therapeutic advantages of medicinal herbs fermented with Lactobacillus plantarum, in topical application and its activities on atopic dermatitis. Phytother Res. 2009; 23:913–919.

9. Segawa S, Fujiya M, Konishi H, Ueno N, Kobayashi N, Shigyo T, et al. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS One. 2011; 6:e23278.

10. Park CW, Youn M, Jung YM, Kim H, Jeong Y, Lee HK, et al. New functional probiotic Lactobacillus sakei probio 65 alleviates atopic symptoms in the mouse. J Med Food. 2008; 11:405–412.

11. Woo SI, Kim JY, Lee YJ, Kim NS, Hahn YS. Effect of Lactobacillus sakei supplementation in children with atopic eczema-dermatitis syndrome. Ann Allergy Asthma Immunol. 2010; 104:343–348.

12. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980; 92:44–47.
13. Smits HH, Engering A, van der Kleij D, de Jong EC, Schipper K, van Capel TM, et al. Selective probiotic bacteria induce IL-10-producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin. J Allergy Clin Immunol. 2005; 115:1260–1267.

14. Hoarau C, Lagaraine C, Martin L, Velge-Roussel F, Lebranchu Y. Supernatant of Bifidobacterium breve induces dendritic cell maturation, activation, and survival through a Toll-like receptor 2 pathway. J Allergy Clin Immunol. 2006; 117:696–702.

15. Isolauri E, Majamaa H, Arvola T, Rantala I, Virtanen E, Arvilommi H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology. 1993; 105:1643–1650.

16. Rosenfeldt V, Benfeldt E, Valerius NH, Paerregaard A, Michaelsen KF. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J Pediatr. 2004; 145:612–616.

17. van der Aa LB, Heymans HS, van Aalderen WM, Sprikkelman AB. Probiotics and prebiotics in atopic dermatitis: review of the theoretical background and clinical evidence. Pediatr Allergy Immunol. 2010; 21:e355–e367.

18. Valdéz JC, Peral MC, Rachid M, Santana M, Perdigón G. Interference of Lactobacillus plantarum with Pseudomonas aeruginosa in vitro and in infected burns: the potential use of probiotics in wound treatment. Clin Microbiol Infect. 2005; 11:472–479.

19. Peral MC, Martinez MA, Valdez JC. Bacteriotherapy with Lactobacillus plantarum in burns. Int Wound J. 2009; 6:73–81.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download