Abstract
Background
The etiology of chronic idiopathic urticaria (CIU) is not completely clear. There are a few antibodies were reported to correlate with CIU.
Methods
The autologous serum skin test (ASST) and allergens were performed. Serum levels of immunoglobulin E (IgE), anti-FcεRI and anti-IgE, anti-Helicobacter pylori (HP) antibodies and anti-thyroglobulin antibody (TGAb) were measured in 100 patients with CIU, acute urticaria (AU) and normal controls respectively.
Results
Eighty-six percent food or inhalant allergens were detected in AU patients, but no allergens were detected in CIU patients and normal controls. Serum anti-FcεRI antibody and anti-IgE antibody levels were higher in the CIU than that in the AU patients and normal controls (p<0.05, respectively). IgE level was lower in the CIU patients (T=190.00, p< 0.05), but increased in the AU patients (T=226.00, p<0.05) compared with the normal controls. The ASST positive rates in the CIU and the AU patients were 53.4% and 12.6% respectively, but all normal controls were negative. The anti-FcεRI antibody level was higher in the ASST-positive CIU patients than those negative ones (T=101.73, p<0.05). In anti-HP antibody positive and TGAb positive CIU patients, anti-FcεRI antibody positive rate was higher than AU patients (p<0.01) and normal controls (p<0.01).
Chronic idiopathic urticaria (CIU) affects 3% to 4% of people and is defined as wheals that recur almost every day, lasting more than 6 weeks. The etiology and pathogenesis of this refractory disease are still uncertain. Some patients suffer from this disease for years or even decades. Regarding its effect on quality of life, CIU is as worse as heart disease1. However, recent findings suggest a role for some antibodies related to CIU, but which of these antibodies is the most important and under what conditions they work remain to be clarified. The purpose of this study was to explore which of these antibodies plays a major role in mast cell degranulation and under what condition this pathogenesis is activated.
Subjects were placed into CIU, acute urticaria (AU), or normal control groups (n=100/group). The subjects were 15 to 63 years old in the CIU group (36 males and 64 females). The subjects were 20 to 65 years old in the AU group (28 males and 72 females). The subjects were 22 to 69 years old in the normal control group (37 males and 63 females). Our subjects were age and sex matched as accurately as possible to decrease errors. Patients with urticaria were chosen from the Department of Dermatology, Yantai Yu Huang Ding Hospital affiliated to the Medical College, Qingdao University. The diagnosis of CIU and AU was made according to the criteria of the European Academy of Allergy and Clinical Immunology (EAACI)2. According to EAACI criteria, the CIU group criteria were: 1. The patient had a history of recurrent wheals over 6 weeks daily or almost daily. 2. No inhalant, food, infection, or drug allergy evidence, and no other definite clinical causes were found. Additionally, physical urticaria, cholinergic urticaria, hereditary angioedema, and urticaria vacuities were excluded. 3. The patients had no history of allergic diseases, such as allergic rhinitis, asthma, or atopic dermatitis, and no history of autoimmune diseases. 4. Antihistamines were not used within 1 week, and steroids or immunosuppressive drugs were not used within 1 month. 5. Subjects were also excluded if exact causes were found during the study follow-up. The selection criteria for the AU group were: 1. The course lasted <6 weeks, and no wheals were observed in the subsequent 6-week follow-up. 2. Exact causes were found. 3. Antihistamine drugs and steroids or immunosuppressant drugs were stopped 1 week and 1 month respectively before the study began. The normal control group criteria were no history of urticaria, asthma, allergic disease, or autoimmune disease. Routine blood and urine tests and liver and kidney function were normal. Women who were pregnant or nursing were excluded. All subjects have allergen screening test before inclusion in the research. The hospital ethics committee agreed to all study procedures.
The autologous serum skin test (ASST) was performed with 50 µl of the patient's own serum intradermally injected into the flexor aspect of the forearm; 50 µl of saline was injected 3 to 5 cm away as a control. The results were measured after 30 minutes. If the serum-injected site manifested a wheal with a diameter at least 1.5 mm greater than that of the saline-injected site, the result was considered positive (Fig. 1)3.
Assays were performed with rat anti-human FcεRI antibody and rat anti-human immunoglobulin E (IgE) antibody enzyme-linked immunosorbent assay kits (Rapidbio, Columbia, CA, USA) according to the manufacturer's instructions. The plates were tested using an automatic quantitative microtiter plate reader (Anthos 2010) at a 450 nm to read absorbance value. The antibody levels were determined according to a standard curve.
Serum IgE level was detected using a protein analyzer (Dade Behring BNII System) according to the manufacturer's instructions.
Serum thyroglobulin antibody (TGAb) was assayed with an E170 MODULAR Immunoassay Analyzer (Roche, Basel, Switzerland). If the absorbance value was >115 IU/ml, the result was considered positive.
A Urease Immunogold Testing kit (Colloidal Gold) was applied to measure serum anti-HP antibody levels according to the manufacturer's instructions.
The data were recorded and processed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The positive rates are expressed as percentages and were analyzed using the χ2 test. Numeric variables are expressed as medians and were analyzed using the Wilcoxon test. A p-value <0.05 was considered significant.
Food or inhalant allergens were detected in most AU patients (positive rate 86%). However, no allergen was observed in CIU patients or in normal controls.
In total, 53 of 100 patients with CIU were positive (positive rate, 53%) and 12 of 100 patients with AU were positive (positive rate, 12%) in the ASST. No positive result was observed in the normal control group. The CIU group ASST-positive rate was significantly higher than that in AU patients and normal controls (χ2=38.31, p<0.01; χ2=72.11, p<0.01).
Serum anti-FcεRI and anti-IgE antibody levels in CIU patients were significantly higher than those in the controls (T=78.00, p<0.05; T=195.00, p<0.05), whereas no difference was observed between AU patients and controls. IgE levels were lower in patients with CIU than those in the controls (T=190.00, p<0.05), but increased IgE levels were observed in AU patients compared with controls (T=226.00, p<0.05). Anti-FcεRI antibody levels were higher in CIU patients than those in the AU group, but IgE levels were lower (T=23.00, p<0.05; T=129.00, p<0.05), Fig. 2, Table 1. Additionally, anti-FcεRI antibody level in ASST-positive CIU patients was higher than that in ASST-negative CIU patients (T=101.73, p<0.05); however, the anti-IgE antibody level was not significantly different between ASST-positive CIU patients and ASST-negative CIU patients (T=312.04, p>0.05; Table 2).
Anti-HP antibody positive rates of the CIU, AU and normal control groups were 29%, 19%, and 23% respectively. TGAb positive rates were 18%, 15%, and 11% respectively. No significant difference in anti-HP antibody or TGAb positive rates was observed among the experimental groups (χ2=2.81, p>0.05; χ2=2.99, p>0.05). The anti-FcεRI antibody positive rate was higher in anti-HP antibody positive CIU patients than that in AU patients (χ2=9.76, p<0.01) and normal controls (χ2=18.33, p<0.01). The anti-FcεRI antibody positive rate was also higher in TGAb positive CIU patients than that in AU patients (χ2=12.58, p<0.01) and normal controls (χ2=12.76, p<0.01). No anti-IgE antibody positive rates were different among the experimental groups (χ2=13.02, p>0.05; Table 3).
Because no allergen could be detected in most CIU patients, the pathogenesis of this disease could not be explained by hypersensitivity type I reactions. The ASST of some CIU patients was positive, indicating that some inflammatory mediators activated mast cells in a non-antigen-mediated manner to induce CIU. Subsequently, it was confirmed that this histamine-releasing mediator was mast cell antibodies, including the anti-FcεRI antibody, anti-IgE antibody, anti-HP antibody, and TGAb4. However, which of these antibodies played the key role and under what condition these antibodies act in CIU pathogenesis were not clarified.
Our research showed that the anti-FcεRI antibody and anti-IgE antibody levels in CIU patients were significantly higher than those in AU patients and normal controls, but no difference was found between AU patients and normal controls. This result indicates that both the anti-FcεRI antibody and anti-IgE antibody play an important role in CIU pathogenesis. Moreover, we found that the anti-FcεRI antibody was higher in the ASST-positive CIU group than that in the ASST-negative CIU group, whereas no significant difference in anti-IgE antibody levels was observed between these two groups, suggesting that the anti-FcεRI antibody may play the key role in ASST-positive CIU. However, the anti-FcεRI antibody and anti-IgE antibody were also detected in normal controls, but normal controls did not manifest CIU symptoms. This result suggests that these autoantibodies could cause CIU only under certain conditions. Anti-IgE antibodies produced first combined with IgE in the patient's serum, resulting in low serum IgE levels in the CIU patients. When IgE level decreased, excessive anti-IgE antibody had the opportunity to bind to IgE on the mast cell surface, leading to IgE cross-linking and activating the tyrosine kinase pathway, causing histamine release. The recombinant humanized anti-IgE monoclonal antibody (omalizumab) reduces free serum IgE levels to treat airway hyper-reactivity. However, this agent has the risk of causing severe urticaria or more serious asthma possibly through the same mechanism. Previous studies have reported that when serum IgE level is low, the FcεRIα subunit could be exposed, providing more opportunity for the anti-FcεRI antibody to bind to FcεRI. This binding activates the tyrosine kinase pathway causing histamine release5.
ASST was first proposed by Grattan et al.6 to detect autoantibodies in CIU patients. The ASST-positive rate of CIU is reported to be 30% to 60%7,8. Some patients meet the CIU diagnosis criteria but show a negative result. Our research confirmed that the anti-FcεRI antibody plays a key role in ASST-positive CIU. We detected allergens using the Immuno CAP 250 system, which can detect 65 kinds of common allergens when screening subjects. Food or inhalant allergens were found in most AU patients (positive rate, 86%), indicating that AU is a type I allergic reaction induced by an allergen. AU and CIU are completely different diseases and have completely different pathogenesis, even though they have same clinical manifestations.
CIU has been correlated with HP infection. The HP infection rate in CIU patients is higher than that of normal controls, indicating that the HP infection was related to CIU9. Bakan et al.10, showed that HP infection induce the generation of anti-FcεRI and anti-IgE antibodies through 'molecular mimicry'. They showed that the Lewis polysaccharide X, Y antigen on the cytomembrane can induce the expression of human leukocyte antigen-DR on gastric mucosal epithelium cells and these cells acted similar to antigen-presenting cells to generate antibodies. However, Zauli et al.11, found no relationship between HP infection and CIU, and the symptoms of CIU did not improve after HP treatment. The anti-HP IgG antibody can be detected in both currently and previously infected patients, and its positive rate is 33% to 36% in the normal population. In this study, no anti-HP antibody positive rate difference was confirmed among the CIU, AU and normal control groups, but anti-FcεRI antibody positive rate was higher in anti-HP antibody positive CIU patients, than that in AU patients and normal controls, which showed that the anti-HP antibody may have an indirect relationship with CIU.
Zauli et al.11. reported that autoimmune thyroiditis is related with CIU. Autoimmune thyroid disease and thyroid antibody in patient with CIU was higher than that in normal controls, indicating that thyroid antibodies might be related with CIU. Some researchers believe that the thyroid auto-antibody could induce histamine release by cross-linking FcεRI on the mast cells surface12. Our study found no TGAb-positive rate difference among the CIU, AU, or normal control groups, but anti-FcεRI antibody positive rate was higher in TGAb positive CIU patients, than that in AU patients and normal controls, indicating an indirect relationship between TGAb and CIU.
CIU is not strictly 'idiopathic' but is also called autoimmune urticaria. The anti-FcεRI and anti-IgE antibodies play the key role in CIU pathogenesis. Contrary to some studies, anti-HP antibody and TGAb have no direct but an indirect correlation with CIU. HP infection and autoimmune thyroid disease may have the same immune background with CIU.
Figures and Tables
Fig. 1
Positive results of Autologous serum skin test: the serum-injected site manifested a wheal and flare with a diameter at least 1.5 mm greater than that of the saline.
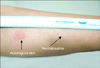
Fig. 2
Serum levels of IgE, anti-FcεRI antibody, and anti-IgE antibody in CIU group, AU group and Normal controls. IgE: immunoglobulin E, CIU: chronic idiopathic urticaria, AU: acute urticaria.

Table 1
The serum levels of IgE, anti-FcεRI antibody, and anti-IgE antibody in CIU and AU patients and normal controls

References
1. Belsito DV. Second-generation antihistamines for the treatment of chronic idiopathic urticaria. J Drugs Dermatol. 2010; 9:503–512.
2. Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau A, et al. Dermatology Section of the European Academy of Allergology and Clinical Immunology. Global Allergy and Asthma European Network. European Dermatology Forum. World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: definition, classification and diagnosis of urticaria. Allergy. 2009; 64:1417–1426.

3. Vohra S, Sharma NL, Mahajan VK. Autologous serum skin test: methodology, interpretation and clinical applications. Indian J Dermatol Venereol Leprol. 2009; 75:545–548.

5. Huilan Z, Runxiang L, Bihua L, Qing G. Role of the subgroups of T, B, natural killer lymphocyte and serum levels of interleukin-15, interleukin-21 and immunoglobulin E in the pathogenesis of urticaria. J Dermatol. 2010; 37:441–447.

6. Grattan CE, Wallington TB, Warin RP, Kennedy CT, Bradfield JW. A serological mediator in chronic idiopathic urticaria--a clinical, immunological and histological evaluation. Br J Dermatol. 1986; 114:583–590.

7. Konstantinou GN, Grattan CE. The autologous serum skin test may be used as a marker for histamine releasing autoantibodies in urticaria and is not relevant to other subject groups. Clin Exp Dermatol. 2009; 34:e473–e474.

8. Sajedi V, Movahedi M, Aghamohammadi A, Gharagozlou M, Shafiei A, Soheili H, et al. Comparison between sensitivity of autologous skin serum test and autologous plasma skin test in patients with Chronic Idiopathic Urticaria for detection of antibody against IgE or IgE receptor (FcεRIα). Iran J Allergy Asthma Immunol. 2011; 10:111–117.
9. Yadav MK, Rishi JP, Nijawan S. Chronic urticaria and Helicobacter pylori. Indian J Med Sci. 2008; 62:157–162.
10. Başkan EB, Türker T, Gülten M, Tunali S. Lack of correlation between Helicobacter pylori infection and autologous serum skin test in chronic idiopathic urticaria. Int J Dermatol. 2005; 44:993–995.

11. Zauli D, Grassi A, Ballardini G, Contestabile S, Zucchini S, Bianchi FB. Thyroid autoimmunity in chronic idiopathic urticaria: implications for therapy. Am J Clin Dermatol. 2002; 3:525–528.
12. Aamir IS, Tauheed S, Majid F, Atif A. Frequency of autoimmune thyroid disease in chronic urticaria. J Coll Physicians Surg Pak. 2010; 20:158–161.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download