Abstract
Skin metastasis of primary gallbladder tumors is extremely rare with a reported incidence of 0.7~9% and it usually involves the thorax, abdomen, the extremities, neck, head region, and scalp. Cutaneous metastasis may occur synchronously or metatochronously. In the present case, the patient had chronic lymphocytic leukemia, which was being treated with an alkylating agent (chlorambucil) when the patient developed skin metastasis from gallbladder adenocarcinoma during post- cholecystectomy follow-up. Given the fact that secondary malignancies occur in chronic lymphocytic leukemia; this clinical setting warrants attention. We aimed to discuss secondary malignancy in chronic lymphocytic leukemia patients and gallbladder adenocarcinoma with skin metastasis, based on a review of the literature and the presented case.
Skin metastasis from visceral malignancies is rare and is an indicator of a poor prognosis. The most common cause of cutaneous metastases is breast cancer, followed by lung, colorectal, renal, ovarian, and bladder cancers1-3. Cutaneous metastasis of malignant gallbladder tumors is extremely rare. Gallbladder carcinoma commonly metastasizes to the liver and regional lymph nodes, and less commonly is associated with extra-abdominal dissemination3. Herein, we present a case in which the patient that had a history of chronic lymphocytic leukemia (CLL) and developed skin metastasis after being diagnosed with gallbladder adenocarcinoma. Based on patient data in the literature, there have been no reports on the development of gallbladder adenocarcinoma after CLL. Herein, we discuss secondary malignancy in a patient with CLL and gallbladder adenocarcinoma in which skin metastasis occurred; this discussion is based on a review of the literature and the presented case.
A 70-year-old male patient was admitted to an emergency unit in December 2009 due to stomachache, nausea, and vomiting, which had exacerbated during the previous 2 days. Cholecystectomy was performed due to cholecystitis. During the operation, it was observed that the patient had a 2 cm gallbladder stone and a tumoral mass originating from the gallbladder corpus with a 1~2 cm satellite lesion. During explorative surgery his liver and peritoneum were intact. Postoperative pathological examination showed a malignant epithelial tumor of the gallbladder; the patient was then referred to our department.
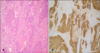
The pathological examination showed patchy epithelial dysplasia and ulcerated regions in the morphologically mostly solid tumor tissue, which formed a patchy gland structure. Immunohistochemical analysis showed diffuse staining with cytokeratin (CK)-7, CK-19, and CK-20, whereas they did not stain with synaptophysin or chromogranin, which was consistent with poorly differentiated primary gallbladder adenocarcinoma. The tumor had infiltrated the entire gallbladder wall and invaded the regional adipose tissue and liver (pT3). One extracted lymph node showed that adenocarcinoma metastasis was present (pN1) (Fig. 1).
The patient had a previous history of B-cell CLL (Rai stage 0/Binet stage A) and had been treated with oral chlorambucil 5 mg/d for the 5 years prior to his presentation to the emergency room on December 2009; the patient was treated concomitantly with oral metoprolol succinate 50 mg in day 1 p.o. and acetylsalicylic acid 150 mg in day 1 p.o. for hypertension and ischemic heart disease. The patient had no family history of cancer, tobacco or alcohol use. The patient's performance, blood pressure, and heart rate were 1 by Eastern Cooperative Oncology Group, 125/85 mmHg and rhythmic 92 bpm; respectively. There was no pathological finding on physical examination except for 1/6 systolic mesocardial murmur and a 1 cm non-tender, mobile and firm lymphadenomegaly (LAM) in the left axillary area. Laboratory data were hemoglobin 10.6 g/dl and hematocrite 30.4%. Other laboratories parameters were within normal range. His chest X-ray was within normal limits. His postoperative carcinoembriogenic antigen (CEA) level was 1.6 (normal range: 0~0.5 ng/ml) and carbohydrate cancer antigen (CA) 19.9 level was 6.6 U/ml (normal range: 0~37 U/ml); Baseline preoperative measurements were not performed.
Thoracic, neck, and abdominal computed tomography (CT) showed multiple bilateral axillary LAMs, of which the largest one was 1×1 cm in dimension and, a 1.2×1 cm right LAM at submandibulary area. The patient did not have distant metastases and was receiving 3 courses of cisplatin (40 mg/m2, D1) and concurrent adjuvant radiotherapy (a dose of 50 Gy) every 21 d.
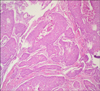
The patient was referred to our surgical department, 7 months post-surgery due to a non-tender, rapidly growing (4~5 d) mass in the inter-scapular region. The lesion, which was 2 cm in diameter, was completely excised, and the patient was again referred to our department. The pathological examination showed a malignant epithelial tumor metastasis in cutaneous mass. Immunohistochemical analysis of the extracted inter-scapular lesion by the pathology department showed diffuse staining with CK-19 and focal staining with CK-7 and 20, and a positive staining pattern with CEA. The tumor cells in the skin exhibited similar morphological properties consistent with gallbladder adenocarcinoma metastasis (Fig. 2).
The patient had no negative pathologic finding except for a couple of 1×1 cm LAMs in the left axillary and submandibulary regions, along with hepatomegaly. Laboratory data were Hb 8.2 g/dl, Hct 29%, leukocyte count 5,400 mm3, serum lactate dehydrogenase 298 U/L (normal range: 125~243 U/L), gamma-glutamyl transpeptidase 278 U/L (normal range: 12~64 U/L). Other laboratories parameters were within normal limits. CEA and CA 19.9 levels were 2.9 ng/ml and 15.4 U/ml, respectively. There was no evidence of activation or blastic transformation of CLL in blood marrow aspiration and biopsy specimens. Abdominal CT showed multiple metastatic lesions, of which the largest was 3.3 cm and located in the 8th segment of the liver's right lobe. On October of 2010 the patient started to receive chemotherapy consisting of cisplatin (80 mg/m2 on d1), calcium folinate (200 mg/m2 on d1), and fluorouracil (1,000 mg/m2 on d1 and d2) with 15 d cycles. However during chemotherapy, new lesions -a 0.5 cm lesion with an accompanying 1 cm red nodular lesion on the scalp, a non-tender, mobile subcutaneous local right scapular lesion, and a 1.5 cm submandibular lesion were observed (Fig. 3).
The patient was admitted to the emergency department 16 days after starting chemotherapy due to mechanical jaundice and pancytopenia. Abdominal ultrasonography and CT showed that the number and size of liver metastases had increased. Percutaneous drainage could not be performed because the intrahepatic biliary branches were not dilated. The patient died due to hepatic failure on the fifth day of hospitalization and 11 months after surgery.
Herein, we present a case of a patient that had a history of CLL with treated with chlorambucil for 5 years prior to current presention and skin metastasis after having been diagnosed with gallbladder adenocarcinoma.
Skin metastasis of visceral carcinomas is rare, with a reported incidence of 0.7~10.4% for all malign tumors3. Cutaneous metastases are usually seen during the later stages of primary cancer and associated with poor prognosis. In some studies the time interval between the diagnosis of the primary tumor and its skin metastasis is >5 years in approximately 7% of cases1,2.
The most common malignancy that metastasizes to skin is breast cancer, which accounts for approximately 24% of all cutaneous metastases, followed by lung, colorectal, renal, ovarian, and bladder cancers, each with a reported incidence of approximately 3.4~4%1,2. In men, lung cancer is the most common cause of skin metastasis, versus breast cancer in women. Other malignant tumors seldom metastasize to the skin. One of these rare cancers is primary malignant tumors of the gallbladder. The cutaneous metastasis of primary gallbladder cancer is extremely rare and has a reported incidence of 0.7~9%1,3,4.
Gallbladder cancers usually infiltrate via direct, lymphatic, vascular, neural, intra-abdominal, and intraductal means, and metastasis to the liver accounts for 76~86% of all cases, whereas metastasis to regional lymph nodes accounts for 60%. The 5-year survival rate among patients diagnosed with gallbladder cancer is <5% and mean survival time in cases of gallbladder cancer with skin metastasis is 7.5 months3. In a study that included 200 patients with cutaneous metastasis, Schoenlaub et al.5 reported that mean survival time was <1 month in 3 patients with skin metastasis and primary gallbladder or liver carcinoma. The presented case died 15 months after diagnosis and 41 d after the first occurrence of metastatic skin lesions.
In total, 86~100% of these cancers are associated with cholelithiasis3. Gallbladder stones may form as a result of carcinoma or may indicate a predisposition for malignancies, as some researchers suggest. In the presented case gallbladder carcinoma was diagnosed following cholecystectomy that was performed for acute cholecystitis due to gallbladder stones.
The most common histological type of gallbladder cancer is adenocarcinoma and based on patient data in the literature, the most common histological type of gallbladder cancer with skin metastasis is adenocarcinoma3.
Cutaneous metastases result from lymphatic or haematogenous spread of primary cancers. Although malignant gallbladder tumors commonly metastasize to thoracic and abdominal skin, the extremities, neck, head, and scalp are sites frequently involved3,6. Typically, the metastatic lesions are non-tender, erythematous, nodular, and either cutaneous or subcutaneous. Padilla et al.7 reported on one patient that had ulcerated epidermal invasion. In various case reports, Padilla et al.7 reported that the lesions were inflammatory skin lesions resembling cellulitis or erythema annulare, condyloma, herpes zoster, epidermal inclusion cysts, and ulcerative lesions other than this typical appearance. In the presented case 0.5~2 cm erythematous mass cutaneous lesions that projected from the skin with a thin peduncle were present and their localization did correspond with data in the literature.
The other significant clinical feature of the presented case is that he was diagnosed with CLL 5 years earlier and was receiving chlorambucil therapy. The incidence of skin cancers had increased 8-fold and other malignancies increase 2-fold in patients with CLL; when compared to similar age and sex groups8. Based on many thorough studies, the incidence of synchronous or subsequent secondary cancer in patients with CLL is 8~19.5%. Second primary tumors occurring with CLL are mostly lung cancer and skin cancers-excluding melanoma and sarcomas. Isolated reports show that late development of various gastrointestinal malignancies, including gastric cancer, colon cancer, and esophageal cancer, occurs in patients with CLL9. According to data in the literature, no CLL patient with accompanying gallbladder carcinoma has been reported.
The occurrence of progressive defects in cellular and humoral immunity, especially in patients diagnosed with B-cell CLL, is obvious. Immune system deficiencies, such as B-lymphocyte defects, hypogammaglobulinemia, and quantitative and functional anomalies of T-cell subtypes, play a role in the development and rapid progression of secondary malignancy in CLL patients9. The role of these mechanisms in secondary primary gallbladder cancer development is not clear9.
Although short-term mortality is still very low in CLL patients that receive treatment, complications regarding therapy or long-standing disease are reported increasingly. In a study by Cheson et al.10, which included the largest number of patients analyzed to date, a significant increase in secondary tumor development, as compared to the general population, occurred in approximately 2,000 recurrent or refractory CLL patients treated with fludarabine. No results showing a risk of secondary malignancy have been reported in studies performed with alkylating agents9,10. Callea et al.9 reported that prolonged chlorambucil treatment in 389 CLL patients did not increase the risk of secondary cancer. The role of target-oriented molecular agents, such as alemtuzumab and rituximab, in secondary cancer development is not clear10. Possible iatrogenic immunosuppressive and mutagenic effects of drugs used for CLL therapy for extended periods of time may increase the risk of secondary tumor development in this group, as compared to the general population. In the present case, the patient had been receiving chlorambucil treatment for the previous 5 years. In spite of this, it is difficult to link CLL therapy with primary gallbladder adenocarcinoma seen in our patient, and this can only be considered a hypothetical opinion that corresponds with data in the literature.
In conclusion, it must be kept in mind that skin metastases can occur in patients with gallbladder adenocarcinomas and may even be the presenting sign. Moreover, it is important to follow-up CLL patients for secondary primary malignancies, such as pulmonary and gastrointestinal system malignancies, using appropriate scanning techniques.
Figures and Tables
References
1. Chopra R, Chhabra S, Samra SG, Thami GP, Punia RP, Mohan H. Cutaneous metastases of internal malignancies: a clinicopathologic study. Indian J Dermatol Venereol Leprol. 2010. 76:125–131.

2. Nashan D, Müller ML, Braun-Falco M, Reichenberger S, Szeimies RM, Bruckner-Tuderman L. Cutaneous metastases of visceral tumours: a review. J Cancer Res Clin Oncol. 2009. 135:1–14.

3. Mukhia R, Shrestha S, Malla K, Sharma VK. Cutaneous metastases from carcinoma of gall bladder. JNMA J Nepal Med Assoc. 2009. 48:318–320.

4. Saeed S, Keehn CA, Morgan MB. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004. 31:419–430.

5. Schoenlaub P, Sarraux A, Grosshans E, Heid E, Cribier B. Survival after cutaneous metastasis: a study of 200 cases. Ann Dermatol Venereol. 2001. 128:1310–1315.
6. Kaur J, Puri T, Julka PK, Gunabushanam G, Iyer VK, Singh MK, et al. Adenocarcinoma of the gall bladder presenting with a cutaneous metastasis. Indian J Dermatol Venereol Leprol. 2006. 72:64–66.

7. Padilla RS, Jarmillo M, Dao A, Chapman W. Cutaneous metastatic adenocarcinoma of gallbladder origin. Arch Dermatol. 1982. 118:515–517.

8. Santoro A, Rilke F, Franchi F, Monfardini S. Primary malignant neoplasms associated with chronic lymphocytic leukemia. Tumori. 1980. 66:431–437.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download