INTRODUCTION
Genetic factors account for the majority of differences in skin color and hair morphology across human ethnic groups. Although many studies have been conducted to examine differences in skin color across populations, few studies have examined differences in hair morphology. However, interest in geographical variations in hair has increased in regards to the cosmetics industry. Previous studies confirmed both morphological and biochemical differences across ethnic groups. Morphological studies have focused mainly on hair color, shape, and responses to external stimuli, while biochemical studies have focused on the compositions of the hair proteins. Only a few studies have assessed the differences in hair lipids across groups. This may be due to the chemical composition of hair, which is composed mainly of keratin protein. Additionally, there are few established methods to examine lipids in human hair.
Hair lipids are composed of fatty acids, cholesterol sulfate, ceramides, and cholesterol. Together, these account for 0.7~1.3% of the total chemical content of hair
1,
2. Integral hair lipids are located in the cell membrane complex (CMC) of hair cuticles, and are important in the maintenance of hair integrity due to qualities including hydrophobicity, moisturization, and stiffness
1,
3. Integral lipids of the hair and epidermal lipids of the skin have similar barrier functions; however, little is known about the nature of population differences in the production of integral hair lipids
1.
Forms of hair damage include physical stress, such as shampooing and combing, and chemical stress, like dyeing and bleaching. The most damaging among these factors is continuous exposure to sunlight. Ultraviolet (UV) light-induced hair damage is difficult to avoid
4,
5. Continuous UV-light exposure results in dryness, roughness, sun-bleaching and breakage due to photo-oxidation
6.
In this study, we examined integral hair lipids in three human populations. The results of UV-induced integral hair lipids differed across groups.
DISCUSSION
The curliness, color, and cross-sectional parameters of hair samples differ across human ethnic groups. However, some proteins, keratins, and molecular structures, including amino acids, have common features across groups
9-
11. Although there are no significant geographical differences in keratin proteins, which are the main structural components of hair, physical characteristics differ among groups. Other components, such as melanin and lipid components, affect the characteristics of hair. Franbourg et al.
9 reported that African hair exhibited less radial swelling when flushed with water compared to Asian or European hair and the authors assumed that there might be differences in hair lipids among human populations. However, proper lipid analysis of this study was not performed. Additionally, Syed et al.
12 reported differences in moisturizing rates associated with the differences in lipid content. In hair care, many experts recognize the importance of moisturization; however, it is difficult to observe integral hair lipids and distinguish them from the lipids produced by sebaceous glands.
The lipid layer is important for maintaining hair integrity; thus, it is also referred to as the "hair barrier". During the process of keratinization in the cortex, an adhesive layer (CMC) forms between adjacent cells through the cortical plasma membrane. CMC is a continuous structure composed of three-layered structures, and the middle layer (δ-layer) is surrounded by inner and outer β-layers. The integral hair lipid is located in the beta-layer
13. The lipid layer of hair is composed mainly of fatty acids, cholesterol sulfate, ceramide, and cholesterol. Orwin
14 observed that globular particles in the intercellular spaces form lamellar structures during hair development. When they migrate to the external area and membrane rupture is occurred, these particles form the lamellar structure. Finally, a lipid layer builds up between the outside of the hair cuticle and the IRS cuticle. One of the most important lipid components is 18-methyleicosanoic acid (18-MEA), which occupies roughly 40% of all fatty acids and has an ester bond or thioester bond with the keratinized cell
15. CMC is a major component of 18-MEA, and it is the lipid layer that decreases friction and increases hydrophobicity of the hair surface
13. We determined that samples from Asians demonstrate higher levels of integral hair lipids than other groups. Free fatty acids, cholesterols, and wax esters were also higher in Asian samples. African samples exhibited higher levels of squalene, but overall, lipid content was lower for this group than for Europeans or Asians. Previous reports
9,
12 using hair swelling revealed that hair lipids of Africans may be more prominent than other ethnic groups. Both our results and previous results may be contradictory to one another. Previously reported results were made from non-standardized hair samples and it may cause the cause for the different results. We used standardized hair samples but there are many factors which can affect the lipid contents that cannot be determined. To analyze more accurate lipid contents, further advanced studies are needed.
UV light is composed of UVA (320~400 nm), UVB (290~320 nm), and UVC (200~290 nm) waves. UVC is blocked by the ozone layer, but UVA and UVB affect skin and hair. Damage to hair due to UV light occurs due to free radicals or cysteic acid, which forms after UV radiation and breaks disulfide bonds
16,
17.
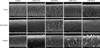
In this study, SEM and TEM analyses showed similar patterns of hair damage, regardless of population origin. As UV irradiation increased, greater focal lifting and loss of the cuticle edge was observed. Furthermore, the number of cuticle layers decreased after 48 hours of UV irradiation. We confirmed that hair surfaces are damaged primarily by UVB light, while hair lipids are damaged primarily by UVA light. These results may be due to differences in the penetration depth of UV light
4.
Damage to the integral lipid layer was similar across groups. Normal hair had a uniform lipid layer, but UV damaged hair displayed swelling of the lipid layer and disruption. The bulging of the lipid layer was mainly observed in samples exposed to UVA irradiation, and the disruption of the continual lipid layer was mainly observed in samples exposed to UVB irradiation.
Lipid content was higher in Asian samples than in other groups, while free fatty acid levels were lower in African samples than in other groups. After UV irradiation, the Asian samples were less damaged than the African samples, while the damage to the European and African samples was time dependent. Thus, we inferred that the quantity of free fatty acid is likely associated with hair damage though there are several additional factors that might also account for the observed effect, such as fiber geometry, diameter and pigment content which need to be investigated from this point forward.
In conclusion, we compared and analyzed the integral hair lipids across three populations and observed morphological changes of hair lipids following UV irradiation. We determined that Asian hair contains more integral hair lipids than European or African hair. After UV irradiation, African and European hair samples exhibited more damage than Asian samples. Therefore, we concluded that integral hair lipids may protect hair against UV light.










 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download