Dear Editor:
Imatinib mesylate, a selective antitumor tyrosine kinase inhibitor, has been approved as the first-line therapy for gastrointestinal stromal cell tumor and chronic myeloid leukemia. Lichenoid drug eruption (LDE) is known to cause uncommon adverse cutaneous reactions of imatinib mesylate, usually appearing with 400 mg or more of imatinib mesylate per day1,2. Here, we present a case of severe LDE after low-dose imatinib mesylate treatment for gastrointestinal stromal cell tumor.
A 77-year-old Korean male patient came to our dermatology clinic in March 2011 for skin rash over the entire body. He was diagnosed with gastrointestinal stromal cell tumor with multiple liver metastasis, and began imatinib with 400 mg per day on September 2007. Two months later, violaceous pruritic papules developed symmetrically on both legs. The skin lesion progressed to the whole body despite a dose reduction to 300 mg per day. His skin lesion was markedly improved after a medication change to sunitinib. However, his kidney function gradually worsened and consequently, sunitinib was changed to imatinib again with a reduced dose to 300 mg per day from December 2010. Extensive skin lesion reappeared soon and a dose reduction to 200 mg per day had no effect on improving skin lesion. On physical examination, violaceous, slightly elevated papules symmetrically covered the extensive body surface (Fig. 1A). The distribution was mainly on both extremities (Fig. 1B). Skin biopsy was performed on his left lower leg. Specimen showed lichenoid lymphocytic infiltration on the upper dermis and deep dermal perivascular lymphocytic infiltration (Fig. 2A). In the high-magnification, 'saw-toothed' rete-ridges were similar to those of lichen planus; however, the finding of cytoid bodies in the granular layer was favorable to LDE (Fig. 2B). Based on the clinical and histologic findings, a diagnosis of LDE due to imatinib was made.
Adverse cutaneous reactions after imatinib are quite common and various2. Among them, imatinib-induced LDE is a relatively uncommon adverse skin reaction and has been reported in 15 cases to date2. In the PubMed online research, all 15 affected patients took more than 400 mg per day of imatinib, except 1 case which showed a lasting LDE despite the dose reduction from 400 mg per day to 200 mg per day3. One case of a Korean patient with LDE after imatinib was reported to date; yet, the case differs with our case in that the eruption was well-controlled with a dose reduction to 300 mg per day4. Ugurel et al.5 reported that imatinib acts as a dose dependent inducer of the development of LDE and may show mild reactivity to low or intermediate dose (200 to 600 mg/day). However, in this case, the patient suffered from extensive drug eruption in spite of the low dose of 200 mg per day. We present here LDE after imatinib treatment of 400 mg per day to a low dose of 200 mg per day. This case presented that a low dose of imatinib does not always prevent LDE and can induce severe eruption in some patients. We suggest clinicians to keep in mind that all patients taking imatinib mesylate have the possibility of extensive LDE even in a low dose and therefore should carry a close observation.
Figures and Tables
Fig. 1
(A) Symmetrically involved erythematous to violaceous papules and plaques, covered extensive body surface area. (B) Slightly elevated violaceous papules and plaque on the right thigh.
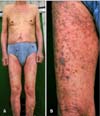
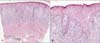
Fig. 2
Histopathological findings. (A) Dense band like lymphocytic infiltration in the upper dermis and deep dermal lymphocytic infiltration (H&E, ×40). (B) Lichenoid lymphocytic infiltration with 'saw-toothed' rete ridges and vacuolar degeneration of the cells in the basal layer. Cytoid bodies in the granular layer (H&E, ×100).
References
1. Kawakami T, Kawanabe T, Soma Y. Cutaneous lichenoid eruption caused by imatinib mesylate in a Japanese patient with chronic myeloid leukaemia. Acta Derm Venereol. 2009; 89:325–326.

2. Amitay-Laish I, Stemmer SM, Lacouture ME. Adverse cutaneous reactions secondary to tyrosine kinase inhibitors including imatinib mesylate, nilotinib, and dasatinib. Dermatol Ther. 2011; 24:386–395.

3. Dalmau J, Peramiquel L, Puig L, Fernández-Figueras MT, Roé E, Alomar A. Imatinib-associated lichenoid eruption: acitretin treatment allows maintained antineoplastic effect. Br J Dermatol. 2006; 154:1213–1216.

4. Yang JH, Shin JW, Kim HD, Park YL, Lee SY, Whang KU. Imatinib mesylate (Gleevec(TM))-induced lichenoid drug eruption improved by tentative dose-reduction and topical steroid. Korean J Dermatol. 2011; 49:155–158.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download