Dear Editor:
In January 2010, the anti-tumor necrosis factor (TNF)-α antibody agents became available for clinical use in Japan for the treatment of psoriasis arthritis1. Here, we report a case of psoriatic arthritis that responded dramatically when the infliximab regimen was switched to adalimumab.
The patient was a 62-year-old Japanese woman. In 2006, the Classification Criteria for Psoriatic Arthritis showed a score of 5 points for the patient, leading to the diagnosis of psoriatic arthritis. In November 2009, the patient was started on infliximab, and dramatic improvements were seen in the rash and joint pains. However, 22 weeks later, the symptoms recurred despite the ongoing therapy. The patient was referred to our hospital in September 2010.
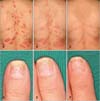
Physical examination revealed hyperkeratotic erythematous plaques on the trunk and extremities. The nails showed onycholysis and pitting of the nail plate. The patient reported joint pain throughout the body and had difficulty walking (Fig. 1A, D). The laboratory examinations yielded the antibodies to infliximab at 40-fold positive and the serum infliximab concentration of ≤0.10 µg/ml.
We thought that the therapeutic effect of infliximab was inadequate and switched to the adalimumab regimen. The administration schedule for adalimumab consisted of an initial dose of 80 mg by subcutaneous injection, followed thereafter by subcutaneous injections of 40 mg at 2-week intervals. The scales had disappeared after 12 weeks, and only faint erythema was apparent (Fig. 1A~C). The nail became flat, and the turbidity disappeared after 12 weeks (Fig. 1D~F). She was able to walk smoothly after 2 weeks. After 4 weeks, the patient felt only the left ankle pain.
The use of the anti-TNF-α antibody agents has resulted in dramatic therapeutic efficacy in patients with refractory psoriatic arthritis2,3. However, scattered reports have also described patients in whom these agents were ineffective or showed reduced efficacy4. Such poor results have been suggested to be due to a failure to maintain effective blood concentrations of the agents following the production of the neutralizing anti-anti-TNF-α antibodies or the accelerated drug metabolism4. While the incidence of antibodies to adalimumab is reportedly at approximately 8%2, the incidence of antibodies to infliximab is reportedly at about 22%3. The higher incidence of the antibodies to infliximab is suggested to be from that the interval between the drug doses is longer for infliximab compared to adalimumab, resulting in a period during which infliximab disappears from the blood, facilitating a production of the neutralizing antibodies5. The present patient experienced a recurrence of the symptoms despite the use of infliximab. Our tests for the reduced efficacy showed that the patient was positive for antibodies to infliximab, leading us to surmise that the blood concentration of infliximab had been reduced by the neutralizing antibodies.
In light of our experience with the patient reported herein, we think that there is a need to actively switch the treatment of psoriasis to a different anti-TNF-α antibody agent when the use of the current agent proves ineffective or shows decreased efficacy. In addition, with few reports having described the psoriasis patients in whom the anti-TNF-α antibody agents were switched, the investigation of a larger number of such patients seem necessary.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download