Abstract
Kaposi sarcoma (KS) is a multicentric proliferative vascular tumor involving the skin and other organs. Human herpesvirus 8 (HHV-8) has been detected in KS lesions and is considered the putative causative agent of KS. The relationship between chronic renal failure, HHV-8, and KS is not clear. KS appears to develop in association with renal transplantation, but is unlikely with dialysis, and there have been few reports on this. Here, we report the case of a 51-year-old man, who underwent peritoneal dialysis to treat chronic renal failure, and presented with multiple brownish plaques on his soles. On histopathological examination, abnormally proliferated vessels, vascular slits, and spindle-shaped cells were seen in the dermis. Immunohistochemical staining for HHV-8 was positive. This case is another example in which factors other than immunosuppression contributed to the development of KS, due to activation of HHV-8.
Kaposi sarcoma (KS) is a rare angiogenic-inflammatory neoplasm that originates from vascular endothelial cells1,2. Human herpesvirus 8 (HHV-8) has been detected in most KS lesions and is considered to be the causative agent. KSoccurs mainly in immunosuppressed patients, such as acquired immunodeficiency syndrome (AIDS) patients or patients receiving immunosuppressive treatment. However, some cases develop in non-immunosuppressed patients.
KS appears to develop in association with kidney transplantation. Transplantation patients are immunosuppressed because of the administration of immunosuppressive agents. By contrast, dialysis patients are not typically immunosuppressed3-8. However, there have been several reports indicating that various immunological abnormalities can lead to impaired immune status in uremic patients9-11.
Here, we present a case of KS in a patient with chronic renal failure undergoing dialysis. We also review the literature on the relationship between KS and dialysis.
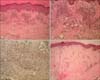
A 51-year-old Korean man visited a dermatology clinic because he developed hyperpigmented lesions on both soles. He had noticed the first lesion 1 year earlier, and it had grown gradually (Fig. 1A). The patient's medical history included a 10-year history of diabetes mellitus and a 3-year history of diabetic nephropathy and hypertension. He had been on regular peritoneal dialysis to treat chronic renal failure for 2 years. In addition, there were swelling, crusts, and scars on both lower extremities that had been diagnosed as stasis dermatitis and treated with topical steroids for 2 years (Fig. 1B). On physical examination, deep gray-colored, non-tender, non-fluctuant linear patches were noted along the arch of the sole, with no ulcerations or nodules. There were reddish-brown patches, edema, and crusts on the shin, and some hyperpigmented crusted plaques on the knee. The laboratory examination, including a complete blood cell count and differential, platelets, fibrinogen, serum electrolytes, chemical analyses, and liver function tests were within normal limits, except for increased blood urea nitrogen (33.1 mg/dl) and creatinine (6.4 mg/dl) levels. Human immunodeficiency virus antibody was negative. Examination for the immune status, such as the Th/Ts ratio in peripheral blood, C3, C4 and immunoglobulin quantization, and sensitization with allergens, was not done. The skin of the sole and shin was biopsied. The specimen from the sole showed numerous dilated, anastomosing slit-like vascular spaces throughout the entire dermis (Fig. 2A). The vascular structures were lined by a single layer of endothelium and there was diffuse extravasation of erythrocytes. Immunohistochemical staining for HHV-8 was strongly positive in the spindle-shaped cells and vascular endothelial cells (Fig. 2B). The specimen from the shin showed proliferating dilated capillaries and fibroblasts with diffuse dermal edema (Fig. 2C). The vascular slits and atypical endothelial cells were absent in this lesion. The sole lesions were diagnosed as KS and the shin lesions as stasis dermatitis.
The sole lesions were treated with cryotherapy and the shin lesions with topical steroid. After one cycle of cryotherapy, the patient was lost to follow-up.
KS has four variants: classical KS, iatrogenic immunosuppression-associated KS, AIDS-associated KS, and African KS1,2. Classical KS usually occurs in the elderly and progresses very slowly. Iatrogenic immunosuppression-associated KS occurs in patients receiving immunosuppressive drugs2. The progression is very rapid and the lesions are multicentric. AIDS-associated KS usually occurs in homosexual patients and is characterized by explosive growth. African KS is characterized by earlier onset and involvement of the internal organs, which usually leads to death. The characteristic histological features of KS are spindle-shaped cell proliferation, an erythrocyte-filled, honeycomb-like pattern of vascular spaces, and small vessels lined with prominent endothelial cells1,2. In our case, the lesions were localized to the sole and the progression was relatively slow. Although the immune status was not evaluated, the patient had no evidence of an immunosuppressed state and there was no history of treatment with immunosuppressive agents. These findings were compatible with the diagnosis of classic KS.
Since Chang et al.12 discovered the DNA of an unknown virus in KS lesions, molecular and epidemiological studies have reported the existence of HHV-8 in KS lesions5. Currently, HHV-8 is considered to be the putative causative agent of KS, meaning that KS is probably derived from a cytokine-driven proliferative process characterized by the mutual stimulation of KS cells and leukocytes7. As the HHV-8 genes induce the deletion of the normal cell cycle check points and block the normal proliferation signal, the result is tumorigenesis2,3. In our case, HHV-8 was documented in the KS lesion immunohistochemically. Nevertheless, in renal transplant recipients, not all patients who carry the virus or who demonstrate seroconversion will develop KS6. A substantial number of patients will carry the virus without developing the tumor. This means that other factors including immune status, and genetic, geographic, and ethnic factors influence the development of KS2,3,12.
KS occurs mainly in kidney transplantation patients, and rarely develops in patients undergoing dialysis3-8. However, dialysis patients are also thought to be susceptible to viruses, such as HHV-8, and might be in danger of developing KS6. Moreover, a recent study showed that the prevalence of antibodies to HHV-8 in patients with chronic renal failure was higher than in normal controls, although the difference was not significant (6.9% vs. 3.88%)3,13.
Dialysis and transplantation patients tend to differ in that the former tend not to be immunosuppressed while the latter are immunosuppressed due to the administration of immunosuppressive agents to prevent graft-versus-host reactions7. However, there have been several reports of various immunological abnormalities leading to impaired immune status in uremic patients. Both cellular and humoral immunity is decreased in uremic patients, although the pathogenesis of this remains unclear. Several hypotheses have been proposed, including a high level of circulating uremic toxins and chronic inflammation dominated by oxidative stress8. Moreover, elevated serum levels of interleukin (IL)-1, IL-6, and growth factors have been documented and these have angiogenic properties11. In favor of this view, the frequency of malignancies other than KS, including liver, colon, rectum, thyroid, kidney, bladder, lung and lymphoid organ malignancies have been reported to be higher in patients undergoing dialysis than in control groups14.
Our patient also had diabetes mellitus and stasis dermatitis. The elevated serum glucose levels that occur in diabetes may alter host immune responses, resulting in an increased predisposition to infectious processes3. Stasis dermatitis occurs in patients with chronic venous insufficiency, and has vasculoproliferative properties. Moreover, an incompetent venous system is thought to lead to the pooling of blood and the resulting reduced flow and reduced oxygen tension could result in immune dysfunction15. These factors might have contributed to the pathogenesis of KS in our patient.
Spindle-cell hemangioendothelioma, pseudo-KS and moderately differentiated angiosarcoma with spindle-cell differentiation are the most important histopathologic simulants of KS. Spindle-cell hemangioendothelioma differs by showing cavernous or widely dilated vascular spaces and collections of epithelioid cells. Pseudo-KS may have spongiosis, more mature small vessels and fewer vascular slits. In addition, detection of HHV 8, positive in KS, and a immunohistochemical study for cutaneous lymphocyte antigen, positive in pseudo-KS, could be helpful in the differential diagnosis2. Angiosarcoma usually shows markedly atypical endothelial cells, and intraluminal shedding of malignant cells, unlike in KS.
The treatment of KS depends on the extent and localization of the lesions, as well as on the clinical type of the disease2. Cryotherapy, ionizing radiation, surgical excision, and photodynamic therapy have been used to treat localized skin lesions. For more aggressive disease, chemotherapy and interferon-α2 have been used.
In conclusion, we documented a case of KS and HHV-8 coexisting in a patient undergoing regular peritoneal dialysis. Our report indicates that factors other than immunosuppression could lead to KS development, due to the activation of HHV-8. We believe that further study of the relationship between dialysis and KS is needed.
Figures and Tables
Fig. 1
(A) A 50-year-old man had a symptomatic hyperpigmented patches along the arches of both feet. (B) There were reddish-brown patches, edema, and crusts on both shins and some hyperpigmented crusted plaques on both knees.
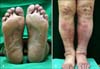
Fig. 2
(A) The biopsy specimen of the sole showed abnormally proliferated and dilated vessels, and vascular slits in the dermis (H&E, ×40). (B) Fascicles of spindle cells intermingled with numerous jagged vascular spaces that were filled with red blood cells (H&E, ×100). (C) Immunohistochemical staining for HHV-8 was positive (HHV-8 stain, ×100). (D) The specimen from the shin showed changes typical of stasis dermatitis, along with the proliferation of capillaries and fibroblasts, while vascular slits and a typical endothelial cells were absent (H&E, ×40). HHV-8: human herpesvirus 8.
References
1. Kim CH, Kim DH, Jeon JS, Kang SG, Kim DW, Cho MK. A case of zosteriform Kaposi's sarcoma after prednisolon treatment. Korean J Dermatol. 2009; 47:583–587.
2. Schwartz RA, Micali G, Nasca MR, Scuderi L. Kaposi sarcoma: a continuing conundrum. J Am Acad Dermatol. 2008; 59:179–206.

3. Yaşar S, Mansur AT, Göktay F, Aydingöz IE. HHV-8 positive Kaposi sarcoma in a patient with end-stage renal disease undergoing hemodialysis: no regressive effect of captopril. Eur J Dermatol. 2007; 17:153–156.
4. Bacha MM, Goucha R, Zouaghi K, Jebali H, Fazaa B, Hedri H, et al. Myeloma, Kaposi's sarcoma and HHV8 infection in hemodialyzed patient. Tunis Med. 2007; 85:237–239.
5. Massanet C, Torguet P, Llistosella E, Casas M. Kaposi sarcoma in an HIV-negative hemodialysis patient. Nefrologia. 2005; 25:341–342.
6. Metaxa-Mariatou V, Chiras T, Loli A, Gazouli M, Vallis D, Nasioulas G. Molecular analysis of Kaposi's sarcoma occurring during haemodialysis. Clin Exp Dermatol. 2004; 29:188–191.

7. Herr H, Kim JU, Kang GH, Moon KC, Koh JK. Kaposi's sarcoma occurring during short-term dialysis: report of two cases. J Korean Med Sci. 2001; 16:130–134.

8. Nampoory MR, Johny KV, Sarkar C, Al-Masry I, Al-Hilali N, Anim JT. The dialysed patient with both castleman disease and Kaposi sarcoma. Nephrol Dial Transplant. 1998; 13:2373–2376.

9. Descamps-Latscha B, Jungers P, Witko-Sarsat V. Immune system dysregulation in uremia: role of oxidative stress. Blood Purif. 2002; 20:481–484.

11. Kato S, Chmielewski M, Honda H, Pecoits-Filho R, Matsuo S, Yuzawa Y, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008; 3:1526–1533.

12. Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994; 266:1865–1869.

13. Almuneef M, Nimjee S, Khoshnood K, Miller G, Rigsby MO. Prevalence of antibodies to human herpesvirus 8 (HHV-8) in Saudi Arabian patients with and without renal failure. Transplantation. 2001; 71:1120–1124.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download