Abstract
Background
Balneotherapy is widely used as an alternative treatment modality for AD. Although the clinical benefit of some mineral waters has been established, their mechanisms of action in alleviating AD are only partly understood.
Objective
The clinical modification and immunomodulatory or anti-inflammatory effects of mineral water from the Suanbo hot springs on the differentiation and cytokine production of Th1, Th2, and regulatory T cells (Treg) were investigated using spleen, skin tissue, and serum from NC/Nga mice.
Methods
The therapeutic effects of bathing in mineral water in a Dermatophagoides farinae body extract ointment (Dfb ointment)-induced AD mouse model were assessed by measuring the modified Scoring atopic dermatitis (SCORAD) index scores, transepidermal water loss (TEWL), histological and immunohistochemical changes of the skin lesion, serum levels of interferon (IFN)-γ, interleukin (IL)-4, IL-5 and immunoglobulin E, mRNA expression of IFN-γ, IL-4 and IL-5 of dorsal skin, and helper T cell differentiation in the spleen.
Results
Bathing in mineral water significantly reduced the modified SCORAD index scores, TEWL, epidermal hyperplasia, and inflammatory cell infiltration. IL-4 production and Th2 cell differentiation showed a decreasing tendency with mineral water bathing, but the Th1 cells did not. On the contrary, differentiation to Treg cells was promoted with mineral water bathing.
Conclusion
Balneotherapy not only has anti-inflammatory activity, but also shows positive effects on cutaneous barrier homeostasis. These results suggest that the favorable effects of balneotherapy may be mediated by modifying the Th2 response, and possibly in part by inducing Treg cell differentiation.
Cutaneous permeability dysfunction and immunologic abnormalities mainly contribute to the pathophysiology of atopic dermatitis (AD), and drive each other in a classic vicious cycle1. Therefore, the treatment of AD should target both of these disease components. Although topical glucocorticoids and systemic therapies improve AD symptoms, there are safety concerns with long-term use. Therefore, it becomes imperative that alternatives or complements to standard therapies be developed to improve this condition.
Balneotherapy is widely used for the treatment of inflammatory skin diseases, such as AD, psoriasis, rosacea, seborrheic dermatitis, and others2,3. Bathing in mineral water is safe and pleasant, and has almost no adverse effects during and after treatment. The therapeutic efficacies and mechanisms of balneotherapy for the treatment of AD have not been fully elucidated, and presumably incorporate chemical, thermal, mechanical and immunomodulatory effects4. Wiedow et al.5 reported that mineral water therapy inhibited Th1 differentiation and cytokine production from keratinocytes, and modulated epidermal Langerhans cells. In a previous study, Haeundae spa therapy decreased the Eczema Area and Severity Index scores and the visual analogue scale for pruritus of adult AD patients6. It was observed that Yonggung oncheon suppressed pro-inflammatory cytokine production in human keratinocyte HaCaT cells, and also modulated the differentiation of CD4 positive cells into Th1, Th2, and Th17 cells, but not regulatory T cells (Treg)7.
In this study, AD-like skin lesions were introduced to NC/Nga mice by repeatedly applying Dermatophagoides farinae body extract ointment (Dfb ointment) and barrier disruption. The existence of active immunomodulatory or anti-inflammatory effects with balneotherapy was then then examined in this AD animal model.
Twenty-eight, four to five-week-old female NC/Nga mice were purchased from Charles River Japan (Yokohama, Japan). The mice were housed and bred under conventional conditions at the animal laboratory of the Uijeongbu St. Mary's Hospital. All animal procedures were approved by the Ethics of Animal Experimentation Committee of Uijeongbu St. Mary's Hospital, and conformed to international standards.
Dfb ointment was prepared by Biostir Inc. (Kobe, Japan). One gram of Dfb ointment contained 41.7 mg of protein, 58.5 µg of Der f 1, and 22.2 µg of Der f 2.
After 1 week of adaptation, the mice were randomly allocated into 4 groups: Group 1, the untreated negative control (CONT); Group 2, Dfb ointment-induced AD as a positive control (AD); Group 3, Dfb ointment+distilled water bathing (AD+DW); and Group 4, Dfb ointment+mineral water bathing (AD+MW). In the first induction, the dorsal hair of the mice was clipped using an electric shaver, and the residual hair was depilated using a hair removal cream. Groups 2 to 4 were challenged by the topical application of 100 mg of Dfb ointment on the shaved dorsal skin and the surface of both ears, while untreated Group 1 served as a negative control. In the second induction, barrier disruption was achieved by 150 µl of 4% sodium dodecyl sulfate treatment on the shaved dorsal skin and the surface of both ears 3 hours before Dfb ointment application. These procedures were repeated 3 to 4 times a week for up to 2 weeks. The experiment schedule is summarized in Fig. 1.
After 2 weeks of Dfb application, when the phenotype of AD-like chronic allergic dermatitis had been established, each of Groups 3 and 4 were immersed in distilled or mineral water baths for 5 minutes (Fig. 2). The temperature of the water was 37℃ to 39℃. Bathing was performed daily for 2 weeks. Group 2 mice were untreated. Suanbo Mineral Water has been classified in the category of waters with low mineral content (total dissolved solids: 348 mg/L), and sodium bicarbonate is the major component. The mean water temperature was 42.40℃, and the pH was 8.39. Compared to other Korean hot springs, Suanbo is richer in its proportions of calcium and sulfate (Table 1). Transepidermal water loss (TEWL) was measured on individual flanks with a skin-evaporative water recorder (Tewameter MPA5®; Courage & Khazawa, Köln, Germany) immediately before bathing at the baseline, on experimental days 1, 3, 4, 7, and 11, and 12 hours after the final bathing. Assessments were carried out in an air-conditioned animal room (20℃ to 21℃; relative humidity 27%).
The severity of dermatitis was grossly assessed by the scoring AD (modified SCORAD) method before bathing at the baseline, on experimental days 3, 7 and 11, and 12 hours after the final bathing. The degree of each symptom was scored as 0 (absence), 1 (mild), 2 (moderate), and 3 (severe). This scoring was based on the severity of 1) erythema/hemorrhage, 2) scaling/dryness, 3) edema, and 4) excoriation/erosion. The sum of the individual scores (minimum 0 and maximum 12) was taken as the dermatitis score. Assessment was performed by an investigator who was blind to the grouping of the animals.
The sampling of blood, skin, and spleen was performed 12 hours after the final bathing. The dorsal skin was excised and divided into two specimens, one for histopathological evaluation and the other for mRNA extraction. Serum samples were stored at -70℃ until analysis. The spleen was processed for flow cytometric analysis.
The skin lesion samples were fixed in 10% formaldehyde, embedded in paraffin, and stained with standard H&E.
Paraformaldehyde-fixed cryosections were incubated overnight at 4℃ with antibodies against mouse anti-CD1 (sc-9161; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-CD4 (sc-70671; Santa Cruz Biotechnology), and anti-CD8 (H-160; Santa Cruz Biotechnology). Immunoreactivity was detected with a peroxidase-conjugated secondary antibody against mouse and rabbit immunoglobulin (Ig) G (DakoCytomation, Glostrup, Denmark), and subsequently counterstained with hematoxylin. Staining with secondary antibodies without primary antibodies was done to rule out non-specific antibody binding.
The serum levels of interferon (IFN)-γ, interleukin (IL)-4, IL-5, and IgE were measured using specific ELISA kits according to the manufacturer's instructions (Mouse IFN-γ: #430804, ELISA MAX™ Deluxe Sets; BioLegend, San Diego, CA, USA, Mouse IgE: #432404, ELISA MAX™ Deluxe Sets; BioLegend, IL-4: #555232, Mouse IL-4 ELISA Set; BD Bioscience, San Diego, CA, USA, IL-5: #555236, Mouse IL-5 ELISA Set; BD Bioscience).
To study the gene expression of the IFN-γ, IL-4, and IL-5 of dorsal skin, total RNA was extracted from the skin using TRIzol (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer's instructions. Total RNA was transcribed into cDNA using a reverse transcription system (Promega, Madison, WI, USA). The primers used for the reverse transcription-polymerase chain reaction (PCR) were as follows: 5'-TTT GCA GCT CTT CCT CAT GG-3' (forward) and 5'-ACC ATC CTT TTG CCA GTT CC-3' (reverse) for IFN-γ, and 5'-ATA TCC ACG GAT GCG ACA AA -3' (forward) and 5'-AAG CCC GAA AGA GTC TCT GC-3' (reverse) for IL-4. For the detection of IL-5, 5'-GAC CTT GAC ACA GCT GTC CG-3' (forward) and 5'-AGC ATT TCC ACA GTA CCC CC-3' (reverse) primers were used. Glyceraldehyde 3-phosphate dehydrogenase genes were used as controls for RNA input. Real-time PCR with SYBR green detection was conducted using a Maxime PCR premix Kit (Intron Biotechnology, Seongnam, Korea) with an ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA, USA) with 50 ng of cDNA.
To analyze Th1, spleen cells were stained with PE anti-mouse CD4 (100407; BioLegend). After cell fixation, pellet cells were washed three times in an 80:20 mixture of fluorescence-activated cell sorting buffer and permeabilization buffer, followed by the suspension of cells in the same buffer mixture with primary antibody for 30 minutes on ice. The permeabilization buffer contained 0.5% saponin and 1% bovine serum albumin in phosphate buffered saline. Subsequently, cells were stained with antigen-presenting cell (APC) anti-mouse IFN-γ (505809; BioLegend). For the analysis of Th2, cells were stained with PerCP anti-mouse CD4 (100431; BioLegend), followed by intracellular staining with Alexa Fluor 488 anti-mouse IL-4 (504111; BioLegend) and PE anti-mouse IL-9 (514103; BioLegend). For the Treg cells, cells were fixed and permeabilized, then stained with Alexa Fluor 488 anti-mouse/rat/human Foxp-3 (320011; BioLegend). Isotype controls were used: PE-Rat IgG2b, κ (for PE anti-mouse CD4), PerCP-Rat IgG2b, κ (for PerCP anti-mouse CD4), APC-Rat IgG1, κ (for APC anti-mouse IFN-γ), Alexa Fluor 488-Rat IgG1, κ (for Alexa Fluor anti-mouse IL-4), PE-Rat IgG1, κ (for PE anti-mouse IL-9), and Alexa Fluor 488-Mouse IgG1, κ (for Alexa Fluor 488 anti-mouse/rat/human Foxp-3). First, the isotype-only tubes under the FL1-versus-FL2 dot-plot with quadrant division were measured. The compensation was adjusted until the cell population gathered into the lower-left quadrant. Data were acquired on a FACS Calibur system (BD Bioscience) and analyzed using CellQuest software (BD Bioscience).
After the repeated application of Dfb ointment for 2 weeks, acute erythematous and edematous dermatitis occurred first, followed by a persistent chronic dermatitis with severe erythema, hemorrhage, scarring, erosion, and excoriation (Fig. 3A). Two weeks after the first challenge, the skin severity scores of the AD group reached a score of more than 9 points (Fig. 3B). Severe scratching behavior of the back and ears with hind paws was also observed. However, in the CONT group, no obvious skin lesions were observed (Fig. 3A). Repeated Dfb application produced marked epidermal hyperplasia, acanthosis, hyperkeratosis, and parakeratosis, accompanied by a prominent inflammatory infiltration in the dermis (Fig. 4A). In parallel with the emergence of epidermal hyperplasia and inflammatory cell infiltration, serum levels of IgE following Dfb ointment application were significantly elevated compared with the CONT group (Fig. 5A). Such Dfb-induced features are representative of AD8.
The efficacy of bathing in mineral water was examined, in Dfb-AD mice, as assessed by changes in clinical appearance, histologic features, inflammatory infiltrates, and basal TEWL. Bathing in mineral water for 2 weeks alleviated the severity of the AD-like skin lesions (Fig. 3A) and markedly reduced dermatitis scores (Fig. 3B) and TEWL (Fig. 3C). Moreover, inflammatory infiltrates also declined after bathing in mineral water (Fig. 4A). The dermatitis scores, TEWL, and inflammatory cell infiltration also declined after bathing in distilled water compared with the AD group, but to a lesser extent than the AD+MW group. The AD group showed no remarkable changes in dermatitis scores, TEWL (Fig. 3B, C), and inflammatory cell infiltration (Fig. 4A).
In parallel with these apparent therapeutic benefits, bathing in mineral water significantly normalized Dfb-induced epidermal hyperplasia (Fig. 4A). In contrast, epidermal hyperplasia, hyperkeratosis, and parkeratosis were still apparent in the AD+DW group (Fig. 4), respectively, compared to the AD+MW group.
The effect of balneotherapy in the infiltration of CD1+, CD4+ and CD8+ cells was further investigated. As shown in Fig. 4B, bathing in mineral water decreased CD1+ cell infiltration. No significant reduction in CD1+ cells was found in the AD+DW group. The effect of balneotherapy in the infiltration of CD4+ and CD8+ cells was also investigated. As shown in Fig. 4C and D, the infiltration of CD4+ and CD8+ cells also remarkably declined after bathing in mineral water. Although the reduction of CD4+ and CD8+ cells in the AD+DW group was also observed, it was not to the same extent as in the AD+MW group.
The total serum IgE levels after 2 weeks of AD induction were markedly higher compared to the CONT group (Fig. 5A). The AD+MW and AD+DW groups failed to reduce total serum IgE levels following Dfb ointment application, and there was an increase in IL-4 levels following Dfb ointment application. Mineral water bathing seemed to suppress Dfb ointment-induced IL-4 levels, unlike distilled water (Fig. 5B). However, no statistically significant difference was found in IL-4 levels between these two groups. There was no significant difference in IFN-γ and IL-5 levels among the CONT and all experimental groups (Fig. 5C, D).
There was a significant increase in the mRNA expression of IFN-γ and IL-4 following AD induction (Fig. 6A, B). The Dfb ointment-induced mRNA expression of IL-4 was reduced by mineral water bathing, but it was not statistically significant (Fig. 6B). However, both the AD+MW and AD+DW groups failed to reduce Dfb ointment-induced IFN-γ mRNA expression (Fig. 6A). No significant difference was found in IL-5 among all the groups (Fig. 6C).
As shown in Fig. 7, mineral water bathing seemed to suppress Dfb ointment-induced Th2 cell proliferation. In contrast to the suppressive effect on Th2 cells, differentiation into Treg cells was promoted with mineral water bathing compared to the AD+DW group. Neither mineral water nor distilled water treatment reduced Dfb ointment-induced Th1 cell proliferation.
Bathing in mineral water markedly improved Dfb ointment-induced AD-like skin lesions grossly and histopathologically in NC/Nga mice. In addition, it significantly normalized the epidermal abnormalities that were induced by Dfb ointment application, such as epidermal thickening and aberrant permeability barrier homeostasis of elevated TEWL. Based on recent studies that showed TEWL to correlate well with disease activity (inflammatory status) in AD, TEWL is a consistent, reproducible, and reliable measure of disease severity in AD9,10. According to Elias et al.1, the normalization of barrier function reduces both cytokine secretion by disrupted keratinocytes and transepidermal penetration of allergens and microbial pathogens, which are the two major drivers of inflammation in AD.
APCs play a pivotal role in the pathophysiology of AD. Dendritic cells (DCs) have the ability to facilitate the recognition, phagocytosis, and transmission of information between the environment and the innate and adaptive immune systems. Impaired skin barrier function allows for the transepidermal penetration of haptens and microbial components, which encounter DCs. Thymic stromal lymphopoietin-stimulated DCs amplify the Th2 immune response, and inflammatory DC subtypes are recruited and predominate in the epidermis and dermis of patients with AD11. In this study, the extensive CD1 positive cell infiltration following Dfb application was largely suppressed by bathing in mineral water. Considering the previous Yonggung oncheon study that demonstrated mineral water to suppress pro-inflammatory cytokine (IL-6 and TNF-α) production and MHC II molecule expression of APCs under toll-like receptor stimulation7, it can be speculated that balneotherapy might modulate inflammatory responses in APCs.
Activated CD4+ T cells lead to the differentiation of Th1 cells and Th2 cells. Th1 cells produce IFN-γ which is well known to be elevated in the chronic phase of AD lesions. Th2 cells secrete IL-4, IL-5, and IL-1312, and among these, IL-4 secretion is increased in the acute phase13. Repeated Dfb ointment application increased the mRNA expression of IFN-γ and IL-4 of dorsal skin, whereas bathing in mineral water altered the mRNA expression of IL-4, but not IFN-γ. Similar findings were also observed in the serum levels of IL-4 and IFN-γ. This result indicates that the suppressive effects of mineral water on AD-like skin lesions in NC/Nga mice are possibly mediated by local tissue Th2 response modification.
IgE hyperproduction is known to cause both acute and chronic AD-like skin symptoms. It was found that total serum IgE levels were remarkably increased by the repeated application of Dfb ointment in NC/Nga mice (Fig. 5A). However, mineral water therapy failed to modulate the Dfb-induced total serum IgE levels. It is speculated that balneotherapy might not be sufficient to initiate the Th2 response and consequently down-regulate serum IgE level.
Treg cells are essential for maintaining peripheral tolerance, preventing autoimmune disease, and limiting inflammatory disease14-17. Foxp3+ Treg cells produce inhibitory cytokines IL-10 or therapeutic gain factor-β as suppressive mediators. Treg cells induce tolerance and suppress both Th1 and Th2 immune responses via these inhibitory cytokines17,18. Suanbo Mineral Water therapy decreased Th2 differentiation and IL-4 expression. The mechanism producing these results still remains unclear. However, mineral water bathing significantly induced Foxp3+ Treg cell differentiation, implicating Treg cell-induced suppression in the immune modulatory effect of mineral water. According to Weiss et al.18 and Hawrylowicz and O'Grarra19, Treg cells produce IL-10, which suppresses the Th2-driven response by inhibiting the proliferation or cytokine production of Th2 cells. It is conceivable that the induction of Treg cells contributed to the suppression of dermatitis, resulting in the subsequent suppression of IL-4.
Both immunological abnormalities and skin barrier dysfunction contribute to the pathogenesis of AD, and thus, balneotherapy seems promising because it not only has anti-inflammatory activity, but also shows positive effects on cutaneous barrier homeostasis. Further research is necessary to elucidate the molecular mechanisms of balneotherapy that modulate cytokine production by CD4 positive T cells and the induction of Treg cells. Investigations to identify the immune-active ingredient of mineral water that is responsible for its therapeutic effects on AD are currently in progress. With the positive results of balneotherapy on an AD mouse model, future plans are under way to evaluate cytokine and T cell profiles in AD patients after balneotherapy.
In this preliminary study, the therapeutic efficacies and immunomodulation of balneotherapy in atopic-like dermatitis was confirmed in NC/Nga mice. To our knowledge, the possible Treg cell-induced immune suppressive effects of balneotherapy in alleviating AD have not been reported previously.
Figures and Tables
 | Fig. 1Schematic diagram of experimental design. AD: atopic dermatitis, TEWL: transepidermal water loss, RT: reverse transcription, PCR: polymerase chain reaction, FACS: fluorescence-activated cell sorting. |
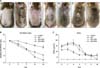 | Fig. 3(A) Photographs of the clinical features of NC/Nga mice (a) in the untreated negative control (CONT), (b, c, d) Dermatophagoides farinae body (Dfb) ointment applied experimental groups on day 14 following the first challenge, (e) Dfb ointment positive control (AD), (f) Dfb ointment+distilled water bathing (AD+DW), and (g) Dfb ointment+mineral water bathing (AD+MW), on day 14 following first bathing. (B) Clinical skin severity scores. (C) Transepidermal water loss (TEWL). Data are presented as the mean±standard deviation. SCORAD: Scoring atopic dermatitis. *p<0.05 vs. AD+DW. |
 | Fig. 4(A) Histological features of dorsal skin (H&E, ×200). Effects of bathing in mineral water on Dermatophagoides farinae body (Dfb) ointment-induced infiltration of CD1+, CD4+, and CD8+ cells in the dorsal skin of NC/Nga mice (B: anti-CD1, C: anti-CD4 and D: anti-CD8; ×100). (a) The untreated negative control, (b) Dfb ointment positive control (AD), (c) Dfb ointment+distilled water bathing (AD+DW), and (d) Dfb ointment+mineral water bathing (AD+MW) on day 14 following the first bathing. |
 | Fig. 5Serum levels of (A) immunoglobulin E (IgE), (B) interleukin (IL)-4, (C) interferon (IFN)-γ, and (D) IL-5 on experimental day 14. Data are presented as the mean±standard deviation. CONT: the untreated negative control, AD: Dfb ointment positive control, AD+DW: Dfb ointment+distilled water bathing, AD+MW: Dfb ointment+mineral water bathing. *p<0.05 vs. CONT. |
 | Fig. 6Effects of bathing in mineral water on Dermatophagoides farinae body (Dfb) ointment-induced mRNA expression of (A) interferon (IFN)-γ, (B) interleukin (IL)-4 and (C) IL-5 of dorsal skin. Data are presented as the mean±standard deviation. CONT: the untreated negative control, AD: Dfb ointment positive control, AD+DW: Dfb ointment+distilled water bathing, AD+MW: Dfb ointment+mineral water bathing. *p<0.05. |
 | Fig. 7Effects of bathing in the mineral water on the Dermatophagoides farinae body (Dfb) ointment-induced T cell profile of spleen cells. Data are presented as the mean±standard deviation. CONT: the untreated negative control, AD: Dfb ointment positive control, AD+DW: Dfb ointment+distilled water bathing, AD+MW: Dfb ointment+mineral water bathing. *p<0.05. |
ACKNOWLEDGMENT
This work was partly supported by the Industry Research Program funded by Chungju, Chungcheongbuk-do, Korea. This manuscript has been presented at the American Academy of Dermatology, the 70th Annual Meeting.
References
1. Elias PM, Hatano Y, Williams ML. Basis for the barrier abnormality in atopic dermatitis: outside-inside-outside pathogenic mechanisms. J Allergy Clin Immunol. 2008; 121:1337–1343.

3. Sukenik S, Abu-Shakra M, Flusser D. Balneotherapy in autoimmune disease. Isr J Med Sci. 1997; 33:258–261.
4. Valitutti S, Castellino F, Musiani P. Effect of sulfurous (thermal) water on T lymphocyte proliferative response. Ann Allergy. 1990; 65:463–468.
5. Wiedow O, Streit V, Christophers E, Ständer M. Liberation of human leukocyte elastase by hypertonic saline baths in psoriasis. Hautarzt. 1989; 40:518–522.
6. Shim WH, Ko HC, Kim BS, Kim MB, Min JA, Kim JW. The efficacy and the safety of Hae-Un-Dae spa therapy for the treatment of adult atopic dermatitis. Korean J Dermatol. 2010; 48:1052–1059.
7. Lee HP, Choi YJ, Cho KA, Woo SY, Yun ST, Lee JT, et al. Effect of spa spring water on cytokine expression in human keratinocyte HaCaT cells and on differentiation of CD4(+) T cells. Ann Dermatol. 2012; 24:324–336.

8. Yamamoto M, Haruna T, Yasui K, Takahashi H, Iduhara M, Takaki S, et al. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol Int. 2007; 56:139–148.

9. Angelova-Fischer I, Bauer A, Hipler UC, Petrov I, Kazandjieva J, Bruckner T, et al. The objective severity assessment of atopic dermatitis (OSAAD) score: validity, reliability and sensitivity in adult patients with atopic dermatitis. Br J Dermatol. 2005; 153:767–773.

10. Sugarman JL, Fluhr JW, Fowler AJ, Bruckner T, Diepgen TL, Williams ML. The objective severity assessment of atopic dermatitis score: an objective measure using permeability barrier function and stratum corneum hydration with computer-assisted estimates for extent of disease. Arch Dermatol. 2003; 139:1417–1422.
11. Novak N. An update on the role of human dendritic cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2012; 129:879–886.

12. Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999; 103:1103–1111.

13. Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996; 383:787–793.

14. Wing K, Sakaguchi S. Regulatory T cells as potential immunotherapy in allergy. Curr Opin Allergy Clin Immunol. 2006; 6:482–488.

15. Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009; 30:636–645.

16. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008; 8:523–532.

17. Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008; 9:239–244.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download