Abstract
Dowling-Degos disease (DDD) is a rare autosomal dominant trait characterized by numerous, symmetrical, progressive and pigmented macules over the axillae, groins, face, neck, arms and trunk as well as scattered comedo-like lesions (dark dot, follicles) and pitted acneiform scars. Histopathology is diagnostic testing using a distinctive form of acanthosis, characterized by an irregular elongation of thin branching rete ridges, with a concentration of melanin at the tips. We report cases of generalized DDD in a single family with autosomal dominant penetrance. DDD can be presented in a generalized form with hypopigmented lesions instead of reticulate hyperpigmentation confined to the flexor areas. This form can be differentiated from DUH by histopathology.
Dowling-Degos disease (DDD) is a rare autosomal dominant trait characterized by numerous, symmetrical, progressive and pigmented macules over the axillae, groins, face, neck, arms and trunk as well as scattered comedo-like lesions (dark dot follicles) and pitted acneiform scars1-3. The disorder usually appears and/or worsens after puberty. DDD, dyschromatosis symmetrica hereditaria (DSH), dyschromatosis universalis hereditaria (DUH) and reticulate acropigmentation of Kitamura (RAPK) share clinical features with each other; yet, they have different pathology findings4-7. Histopathology is diagnostic testing using a distinctive form of acanthosis, characterized by an irregular elongation of thin branching rete ridges, with a concentration of melanin at the tips8,9. Recently, the loss-of-function in the keratin 5 (KRT5) gene and a gene locus mapping to chromosome 17p13.3 have been described recently in cases of DDD10,11. In this study, we report a family with autosomal dominant inheritance (Fig. 1) of a skin disorder with clinical features of generalized DDD (Fig. 2, 3, 4).
The proband was an 82-year-old man who was presented with progressively generalized skin lesions since adolescence. He began to develop areas of hyperpigmentation, initially on the face, but then soon affecting the neck, axillae, groin, trunk and limbs (Fig. 2). The eruption has continued to become more extensive throughout adulthood leading to generalized pruritus. The patient was born of a non-consanguineous marriage. All of his offspring were affected with the same disease. His general physical examination was normal. Skin examination revealed a symmetrical reticulate hyperpigmentation predominantly affecting the lower face, skin folds, major flexures, back and legs. Symmetrically distributed reticulate hypopigmentation was also seen in the legs. Multiple pits varying in size from 1~3 mm and open comedone-like lesions were distributed over the cheeks, perioral area, neck and back. No breaks in the epidermal ridge pattern on the palms and soles were seen. The mucosa, nails, teeth and hair were normal. On investigation, complete blood count, blood chemistry, liver and renal function tests were all within normal limits.
A biopsy was conducted from a hyperpigmented lesion over the shin, and histopathological examination revealed hyperkeratosis, acanthosis and irregular elongated thin branching rete ridges growing down into the dermis and increased melanin pigment in the lower part of the rete pegs (Fig. 5). Another skin biopsy from comedo-like lesions on the back shows keratin-filled cysts resembling comedones (Fig. 5). These features were entirely consistent with the clinical diagnosis of DDD8,9.
A 58-year-old man (first son of the proband) was presented with reticulate hyperpigmented skin lesions over the face, chest, back, axillae and flexures since adolescence. Initially, the lesions, which first started over the face, were raised above the skin surface and were small. Gradually, these lesions progressed to form pits filled with blackish material. He developed similar lesions over the back and chest over the next year. There was no history of other skin diseases. His father, all his siblings and their offspring were affected with the same disease. His general physical examination was within normal limits. Skin examination revealed open comedone-like lesions and multiple pits of varying sizes distributed over the face, neck and limbs. The face and neck were more severely affected when compared to the trunk and limbs. There was diffuse reticulate pigmentation over the face, axillae, inguinal region and back. The rest of the skin examination was unremarkable.
A 50-year-old woman, the younger sister of the patient in case 2, had progressively generalized skin lesions after puberty. Her skin lesions were very similar to his brother's lesions, with generalized reticulate hyperpigmentation, open comedo-like lesions and multiple pits of varying sizes on the face, perioral area, flexures and axillae. There was no history of inflammatory skin diseases prior to the onset of the lesion. There was no significant past medical, surgical and gynecological history. Skin examination revealed multiple pits of varying sizes distributed over the face, upper back, upper chest, arms, cubital fossa and over forearms. There was diffuse reticulate pigmentation over the cubital fossa, axillae, inguinal region and face. The rest of the skin examination was unremarkable. Her general physical examination and all relevant investigations were within the normal limit. We did not perform a genetic study in our patients due to their religious belief.
DDD has characteristics of clinical and histopathological features. The diagnosis is very simple when classical features are present, as observed in our cases. We had considered the differential diagnosis of familial dyskeratotic comedones, DSH, DUH and RAPK, which share some clinical features with each other but have different pathology findings4-7. In our cases, the proband, his offspring and niece had generalized DDD. The generalized DDD has the typical feature of classic DDD (reticulate hyperpigmentation at flexural area) and generalized reticulate hyperpigmentation at the trunk, limbs and other cutaneous findings, including reticulate hypopigmented macules, facial pits, comedo-like papules and palmar pits. Histopathology is a diagnostic testing with a distinctive form of acanthosis, characterized by downward elongations of thin rete ridges with reticulated or fenestrated patterns, with a concentration of melanin at the tips and occasional follicular plugging and horn cysts8,9.
Due to the overlap of clinical features, there is debate as to whether DDD, DUH and RAPK are separate entities or represent a spectrum of a single disorder. RAPK is characterized by atrophic pigmented spots on the back of the hands and feet and palmar pits. These lesions may or may not be observed in patients with DDD12-15. These findings were not present in our patients. DUH (a generalized disorder) and DSH (a localized disorder) are characterized by diffuse symmetrically distributed hypopigmented macules or papules mixed with hyperpigmentation. In DSH, the pigmentary changes are confined to the back aspects of the hands and feet16.
The genetic defect of DDD has not yet been well defined. A recently reported series described the loss of-function mutations in the KRT5 gene10,11. These data confirm that haploinsufficiency for K5 engenders an excess of unpaired, soluble K14 that is then responsible for DDD and points to a prominent role for the keratin intermediate filament cytoskeleton within the basal keratinocytes in epidermal pigment biology17-19. Another genetic defect of DDD has been reported in the gene locus mapping to chromosome 17p13.3 and chromosome 1q21 with pathogenic mutations located in the DSRAD gene11.
In order to categorize these different disorders with overlapping clinical or histopathologic features, it seems that there are two major groups20. The first is DDD, which has the characteristic histologic findings of elongated rete ridges, horn cyst formation and hyperpigmented tips. The distribution may be reticulate in flexural areas (classic DDD) or generalized with hypopigmented papules (generalized DDD), and it may be distinguished by the presence of acantholytic cells (Galli-Galli disease). The second group is dyschromatosis, including DUH and DSH. Clinically, both have hyperpigmented and hypopigmented lesions and histopathologic findings which are different from those of the DDD group.
In conclusion, we report cases of generalized DDD in a single family with autosomal dominant penetrance. DDD can be presented in a generalized form with hypopigmented lesions instead of reticulate hyperpigmentation confined to the flexor areas. This form can be differentiated from DUH by histopathology.
Figures and Tables
Fig. 1
Pedigree of family with generalized Dowling-Degos disease (DDD). Proband (P), his first son (case 2), and his second daughter (case 3) had generalized DDD.
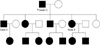
Fig. 2
Skin lesion of proband, 82-year-old man. (A, B) Reticulate hyperpigmentation, multiple pits and comedo-like lesions distributed over the face, perioral area and neck. (C) Reticulate hyperpigmentation with symmetrically distributed hypopigmented macules on shins. (D) Comedo-like lesions distributed over the back.

Fig. 3
Son of proband, 58-year-old man. (A) Reticulate pigmented macules, multiple pits and comedo-like lesions distributed over the face, perioral area and neck. (B) Reticulate hyperpigmentation on axilla.
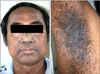
Fig. 4
Daughter of proband, 50-year-old woman. (A) Reticulate pigmented macules, multiple pits and comedo-like lesions distributed over the face, perioral area and neck. (B, C) Reticulate hyperpigmentation on forearms.
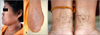
Fig. 5
Skin biopsy specimens from proband of hyperigmented macule (A) and comedo-like lesion (B). (A) Epidermal hyperkeratosis, acanthosis, irregular elongated thin branching rete ridges growing down into the dermis and increased melanin pigment in the lower part of rete pegs (H&E, ×200). (B) Keratin-filled cysts resembling comedones with irregular elongated thin branching rete ridges growing down into the dermis and increased melanin pigment in the lower part of rete pegs (H&E, ×40).

References
1. Crovato F, Nazzari G, Rebora A. Dowling-Degos disease (reticulate pigmented anomaly of the flexures) is an autosomal dominant condition. Br J Dermatol. 1983; 108:473–476.

3. Kim YC, Davis MD, Schanbacher CF, Su WP. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): a clinical and histopathologic study of 6 cases. J Am Acad Dermatol. 1999; 40:462–467.

4. Lestringant GG, Masouyé I, Frossard PM, Adeghate E, Galadari IH. Co-existence of leukoderma with features of Dowling-Degos disease: reticulate acropigmentation of Kitamura spectrum in five unrelated patients. Dermatology. 1997; 195:337–343.

5. Ostlere L, Holden CA. Dowling-Degos disease associated with Kitamura's reticulate acropigmentation. Clin Exp Dermatol. 1994; 19:492–495.

6. Sandhu K, Saraswat A, Kanwar AJ. Dowling-Degos disease with dyschromatosis universalis hereditaria-like pigmentation in a family. J Eur Acad Dermatol Venereol. 2004; 18:702–704.

7. Thami GP, Jaswal R, Kanwar AJ, Radotra BD, Singh IP. Overlap of reticulate acropigmentation of Kitamura, acropigmentation of Dohi and Dowling-Degos disease in four generations. Dermatology. 1998; 196:350–351.

8. Harper JI, Trembath RC. Genetics and genodermatoses. In : Rook AJ, Burns T, editors. Rook's textbook of dermatology. 7th ed. Malden, Mass: Blackwell Science;2004. p. 15.11–15.13.
9. Howell JB, Freeman RG. Reticular pigmented anomaly of the flexures. Arch Dermatol. 1978; 114:400–403.

10. Betz RC, Planko L, Eigelshoven S, Hanneken S, Pasternack SM, Bussow H, et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am J Hum Genet. 2006; 78:510–519.

11. Li CR, Xing QH, Li M, Qin W, Yue XZ, Zhang XJ, et al. A gene locus responsible for reticulate pigmented anomaly of the flexures maps to chromosome 17p13.3. J Invest Dermatol. 2006; 126:1297–1301.

12. Crovato F, Desirello G, Rebora A. Is Dowling-Degos disease the same disease as Kitamura's reticulate acropigmentation? Br J Dermatol. 1983; 109:105–110.

13. Berth-Jones J, Graham-Brown RA. A family with Dowling Degos disease showing features of Kitamura's reticulate acropigmentation. Br J Dermatol. 1989; 120:463–466.

14. Cox NH, Long E. Dowling-Degos disease and Kitamura's reticulate acropigmentation: support for the concept of a single disease. Br J Dermatol. 1991; 125:169–171.

15. Al Hawsawi K, Al Aboud K, Alfadley A, Al Aboud D. Reticulate acropigmentation of Kitamura-Dowling Degos disease overlap: a case report. Int J Dermatol. 2002; 41:518–520.

16. Oyama M, Shimizu H, Ohata Y, Tajima S, Nishikawa T. Dyschromatosis symmetrica hereditaria (reticulate acropigmentation of Dohi): report of a Japanese family with the condition and a literature review of 185 cases. Br J Dermatol. 1999; 140:491–496.

17. Bonifas JM, Rothman AL, Epstein EH Jr. Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science. 1991; 254:1202–1205.

18. Bonifas JM, Bare JW, Lynch ED, Lebo RV, Epstein EH Jr. Regional assignment of the human keratin 5 (KRT5) gene to chromosome 12q near D12S14 by PCR analysis of somatic cell hybrids and multicolor in situ hybridization. Genomics. 1992; 13:452–454.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download