Abstract
Background
The fibrous proteins of extracellular matrix (ECM) produced by dermal fibroblast contributes to the maintenance of connective tissue integrity.
Objective
This study is carried out to identify the bioactive ingredient from natural products that enhances ECM production in dermal fibroblasts.
Methods
Bioassay-directed fractionation was used to isolate the active ingredient from natural extracts. The effects of rasatiol (isolated from Raphanus sativus) on ECM production in primary cultured human dermal fibroblasts was investigated by enzyme linked immunosorbent assay and western blot analysis.
Results
Rasatiol accelerated fibroblast growth in a dose-dependent manner and increased the production of type 1 collagen, fibronectin and elastin. Phosphorylation of p42/44 extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and Akt was remarkably increased by rasatiol, indicating that enhanced ECM production is linked to the activation of intracellular signaling cascades.
The extracellular matrix (ECM) is the largest component of the dermal skin layer and is responsible for skin structure, in terms of providing tensile strength and elasticity. The ECM is composed of a variety of fibrous structural proteins, including collagens, proteoglycans, glycoproteins, and polysaccharides1. In the dermis, most of the ECM proteins are synthesized from fibroblasts2. The importance of ECM in maintaining the skin texture is evident in the collapse of dermal connective tissues and reduction of ECM concurrent with skin aging signs, such as wrinkle formation and reduction of elasticity3. Therefore, it has long been believed that the enhancement of fibroblast activity, in the context of ECM production, may be a key feature to maintaining healthy skin textures.
Many researchers have tried to develop bioactive materials that can be used for enhancing fibroblast activities. One promising methodology still being widely adopted is the screening of natural extracts and isolating the active compounds from them. In the course of screening bioactive materials for stimulating collagen synthesis from natural compounds, we isolated a novel compound rasatiol from the seed of Raphanus sativus, called 'NaBokJa' in herbal medicine. It is shown that rasatiol has a potential to increase type I collagen synthesis in dermal fibroblasts cultured in vitro. In addition, we demonstrated that the stimulatory effects of rasatiol on type I collagen synthesis is mediated through the activation of mitogen-activated protein kinase (MAPK) signaling pathways.
Normal human skin samples were obtained from circumcisions in accordance with the ethical committee approval process of Chungnam National University Hospital. Specimens were briefly sterilized in 70% ethanol, minced, and then incubated in DMEM supplemented with 10% fetal bovine serum and antibiotics (Gibco BRL, Rockville, MD, USA). Dermal fibroblasts were produced from explants after 5~7 days. At confluence, cells were routinely subcultured using a 1 : 4 split ratio. Cells were used between passages 4~10. For treatment with rasatiol, approximately 1×106 cells were seeded on 100-mm culture dishes and grown to sub-confluence. Cells were serum-starved for 24 hours, then treated with rasatiol in a serum-free medium.
We isolated rasatiol from the seeds of R. sativus (Kyongdong Herbal Market, Seoul, Korea) using bioassay-directed fractionation. Air-dried seeds of R. sativus (3 kg) was cut into pieces and extracted with 5 volumes of 80% ethanol for 5 days at room temperature. The extract was evaporated under reduced pressure conditions, and then subsequently, fractionated in n-hexane, chloroform, butanol and finally in water. The n-butanol-dissolved fraction was then separated into 5 sub-fractions by silica gel chromatography using a gradient developing solution (chloroform : methanol, 100 : 1→1/1). A third sub-fraction was rechromatographed on silica gel using a gradient developing solution (chloroform : methanol, 20 : 1→1/1), and then divided into four additional sub-fractions. Of these, the fourth was recrystallized using ethanol (Fig. 1A) to give a new crystalline compound (250 mg) named as rasatiol because this is a sugar-containing chemical isolated from R. sativus. The structure of rasatiol was determined by using various nuclear magnetic resonance (NMR) spectroscopic methods, and electrospray ionization mass spectrometry (ESI-MS) (Fig. 1B)4.
Fibroblast cells were seeded in a 60-mm culture dish and treated with 1 µCi of [3H]thymidine (Amersham, Buckinghamshire, UK) for a [3H]thymidine uptake assay. Following incubation on the indicated time point, cells were washed twice with phosphate buffered saline and incubated with 0.1 N NaOH at room temperature. Radioactivity in cell lysates was measured using a liquid scintillation counter.
ELISA kit for type 1 procollagen was purchased from Takara Bio Inc. (Otsu, Shiga, Japan), and fibronectin from American Diagnostica (Greenwich, CT, USA). Levels of type 1 procollagen and fibronectin secreted from fibroblasts were quantified according to the manufacturer's recommended protocols. Measurements were repeated at least three times, with independent cell batches.
Cells were lysed in Proprep solution (Intron, Daejeon, Korea). After vigorous pipetting, extracts were centrifuged for 15 minutes at 13,000 rpm. Total protein was measured using a Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Samples were run on SDS-polyacrylamide gels, transferred onto nitrocellulose membranes, and incubated with appropriate antibodies overnight at 4℃ with gentle agitation. Blots were then incubated with peroxidase-conjugated secondary antibodies for 30 minutes at room temperature, and visualized using enhanced chemiluminescence (Intron). The following primary antibodies were used in this study: collagen type 1 α1 and elastin (Santa Cruz Biotechnologies, Santa Cruz, CA, USA); phospho-p38 MAPK, total-p38 MAPK, phospho-p42/44 extracellular signal-regulated kinase (ERK), total-p42/44 ERK, phospho-Akt, total-Akt (Cell Signaling Technology, Danvers, MA, USA); actin (Sigma, St. Louis, MO, USA).
In a preliminary attempt to screen the putative fibroblast activators, we used the polarity-based sequential fractionation method. This process involved two step chromatography based on a silica matrix, and one step recrystallization (Fig. 1A). Results of 1H-NMR, 13C-NMR, and ESI-MS showed that the crystallized active ingredient from the seeds of R. sativus is an unprecedented compound named rasatiol (Fig. 1B). The purity of rasatiol obtained using the above-mentioned method was approximately 99% by high performance liquid chromatography.
To investigate the effect of rasatiol on the growth of fibroblasts, we first performed a [3H] thymidine uptake assay. Dermal fibroblasts were treated with various concentrations of rasatiol and incubated for 2 d. As shown in Fig. 2, rasatiol increased the [3H] thymidine uptake of fibroblasts in a dose-dependent manner.
Since the proliferative property of fibroblasts is frequently linked to ECM production in fibroblasts, we next determined the effects of rasatiol on the production of collagen, fibronectin, and elastin. An ELISA assay showed that rasatiol increased secretion of type 1 procollagen and fibronectin in a dose-dependent manner, and the effects were comparable with a positive control ascorbic acid (Fig, 3A, B). Consistent with this result, western blot analysis showed that rasatiol increased intracellular protein level of collagen type 1 α1 (Fig. 3C). Rasatiol also slightly increased the production of elastin, suggesting that rasatiol has a potential to activate fibroblasts.
As the intracellular signaling cascades p42/44 ERK and p38 MAPK are implicated in the regulation of ECM production5,6, we investigated the effects of rasatiol on MAPK activation. As shown in Fig. 4, rasatiol treatment led to phosphorylation of p42/44 ERK and p38 MAPK. In addition, the phosphorylation level of Akt, an important signaling molecule for cell survival7, was also increased by treatment with rasatiol. These results suggest that rasatiol increases ECM production via the intracellular MAPK signaling cascades.
We isolated a novel compound named as rasatiol from R. sativus. The rasatiol is an unprecedented compound in which two syringic acid moieties are linked to a disaccharide group. In previous reports, syringic acid has been shown to possess several biological activities, such as the inhibition of COX-2 activity, antioxidant potential, and hepatoprotective activity8,9. However, the rasatiol did not indicate such biological activities in this study (data not shown). Elucidating the similar potentials of rasatiol as comparing to syringic acids will be an interesting future study.
In this study, we demonstrated that rasatiol has a potential to enhance ECM synthesis in cultured dermal fibroblasts. Rasatiol increased the production of type 1 collagen, elastin, and fibronectin as well as enhanced fibroblast proliferation. In addition, the rasatiol effect was linked to activation of the ERK1/2, p38 MAPK, and Akt intracellular signaling pathway.
The ECM is the largest component of the dermal skin layer and the synthesis of ECM is a key feature for providing the tensile strength of skin. The ECM consists primarily of fibrous proteins and complex sugars, which form fibrillar networks and a ground substance. As skin texture is largely dependent on ECM dynamics, the important role of dermal fibroblasts should be appreciated in this setting. For example, the population doubling-time of older cells was longer than that of the younger ones10, and the intracellular expression of procollagen protein in fibroblast of young skin is greater than that of the aged one11. In line with these, older skin shows less elasticity and more wrinkles. Therefore, activation of cell proliferation and enhancement of collagen production to strengthen the skin texture is an attractive approach for treating connective tissue-related skin problems, such as wrinkle formations.
Type I collagen is mainly synthesized from dermal fibroblasts as precursor molecules called pro-collagen and then processed to mature collagen, providing major constituent of ECM. Elastin and fibronectin also play a pivotal role in determining the mechanical strengths of the skin. Elastin is also secreted from skin fibroblasts as a soluble precursor tropoelastin, and subsequently assembles into insoluble fiber, which is crucial for the resilience and elasticity of skin12. Fibronectin is secreted from various cell types including fibroblasts, and plays a critical role in the wound healing and mechanical tension generation process. Not only it serves to bind different components of the ECM together, but also it act as sites on the ECM to which cells can bind13. Because rasatiol showed a potential to enhance collagen, elastin and fibronectin in dermal fibroblasts, it could be suggested that rasatiol can be used to strengthen skin textures and treat connective tissue-related skin problems.
A number of intracellular signal transduction pathways have been reported to play a role in regulation of many cellular functions. Gene expression is also regulated by an intracellular signaling system. As for collagen expression, the intracellular signaling cascades including MAPK, Akt, and SMAD, are involved in the regulation processes. The primary role of MAPK has been previously recognized in pro-collagen synthesis14-16. And, the Akt has dual profibrotic effects, increasing collagen synthesis and decreasing its degradation via down-regulations of MMP-117. We have demonstrated that rasatiol significantly increases the phosphorylation of ERK1/2, p38 MAPK and Akt. Based on the association of collagen synthesis with the activation of MAPK, the rasatiol apparently acts as an intracellular signal modulator.
In summary, we isolated rasatiol from R. sativus, and demonstrated that rasatiol stimulates collagen production in dermal fibroblasts. Our results suggest that rasatiol could be used for connective tissue-related skin problems as a co-modality together with first-line treatments.
Figures and Tables
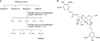 | Fig. 1(A) Isolation of rasatiol from Raphanus sativus. (B) The chemical structure of rasatiol. Rasatiol was isolated from the seeds of R. sativus using bioassay-directed fractionation. After solvent fractionation, two applications of column chromatography followed by recrystallization were used to isolate the active compound. The purity of rasatiol was confirmed to be ~99%, as determined using high performance liquid chromatography, nuclear magnetic resonance, and electrospray ionization mass spectrometry studies. |
 | Fig. 2Effects of rasatiol on the growth of dermal fibroblasts. Cells were treated with rasatiol at the indicated concentrations for 2 d in the presence of [3H]thymidine. Radioactivity was measured using a liquid scintillation counter. Rasatiol increased the [3H]thymidine uptake of fibroblasts in a dose-dependent manner. Results are shown as a percentage of the control±standard deviation (*p<0.05 versus control). |
 | Fig. 3Effects of rasatiol on collagen and elastin production in dermal fibroblasts. (A, B) Cells were treated with rasatiol at the indicated concentrations for 2 d. A conditioned medium was collected, and the amounts of secreted procollagen type 1 and fibronectin were measured using ELISA. Rasatiol increased secretion of type 1 procollagen and fibronectin in a dose-dependent manner, and the effects were comparable with a positive control ascorbic acid. Results are shown as a percentage of a control±the standard deviation (*p<0.05 versus control). (C) Cellular proteins were harvested and the protein levels for collagen type 1 α1 and elastin were verified using western blot analysis. Consistent with ELISA results, intracellular protein level of collagen type 1 α1 was increased in western blot analysis. Rasatiol also slightly increased the production of elastin. ELISA: enzyme-linked immunosorbent assay, Vit.: vitamin. |
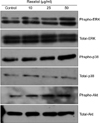 | Fig. 4Effects of rasatiol on intracellular signaling pathways. Cells were treated with rasatiol for the indicated times. Cellular proteins were prepared and phosphorylations of ERK, p38 MAPK, and Akt were determined using western blot analysis. Rasatiol treatment led to phosphorylation (phspho) of p42/44 ERK and p38 MAPK. In addition, the phosphorylation level of Akt, was also increased by treatment with rasatiol. ERK: extracellular signal-regulated kinase, MAPK: mitogen-activated protein kinase. |
ACKNOWLEDGMENT
This study was supported by a grant of the Korea SMBA R&D Project, Small and Medium Business Administration, Republic of Korea (Grant No. SM122106).
References
1. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010; 123:4195–4200.

2. Pieraggi MT, Bouissou H, Angelier C, Uhart D, Magnol JP, Kokolo J. The fibroblast. Ann Pathol. 1985; 5:65–76.
3. Uitto J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol Clin. 1986; 4:433–446.
4. Ignotz RA, Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986; 261:4337–4345.

5. Lim IJ, Phan TT, Tan EK, Nguyen TT, Tran E, Longaker MT, et al. Synchronous activation of ERK and phosphatidylinositol 3-kinase pathways is required for collagen and extracellular matrix production in keloids. J Biol Chem. 2003; 278:40851–40858.

6. Lee DJ, Rosenfeldt H, Grinnell F. Activation of ERK and p38 MAP kinases in human fibroblasts during collagen matrix contraction. Exp Cell Res. 2000; 257:190–197.

7. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999; 96:857–868.

8. Stanikunaite R, Khan SI, Trappe JM, Ross SA. Cyclooxygenase-2 inhibitory and antioxidant compounds from the truffle Elaphomyces granulatus. Phytother Res. 2009; 23:575–578.

9. Itoh A, Isoda K, Kondoh M, Kawase M, Kobayashi M, Tamesada M, et al. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol Pharm Bull. 2009; 32:1215–1219.

10. Yoon IK, Kim HK, Kim YK, Song IH, Kim W, Kim S, et al. Exploration of replicative senescence-associated genes in human dermal fibroblasts by cDNA microarray technology. Exp Gerontol. 2004; 39:1369–1378.

11. Cristofalo VJ, Pignolo RJ. Replicative senescence of human fibroblast-like cells in culture. Physiol Rev. 1993; 73:617–638.

12. Sephel GC, Davidson JM. Elastin production in human skin fibroblast cultures and its decline with age. J Invest Dermatol. 1986; 86:279–285.

13. Gildner CD, Lerner AL, Hocking DC. Fibronectin matrix polymerization increases tensile strength of model tissue. Am J Physiol Heart Circ Physiol. 2004; 287:H46–H53.

14. Nagai Y, Miyata K, Sun GP, Rahman M, Kimura S, Miyatake A, et al. Aldosterone stimulates collagen gene expression and synthesis via activation of ERK1/2 in rat renal fibroblasts. Hypertension. 2005; 46:1039–1045.

15. Sato M, Shegogue D, Gore EA, Smith EA, McDermott PJ, Trojanowska M. Role of p38 MAPK in transforming growth factor beta stimulation of collagen production by scleroderma and healthy dermal fibroblasts. J Invest Dermatol. 2002; 118:704–711.

16. Rodríguez-Barbero A, Obreo J, Yuste L, Montero JC, Rodríguez-Peña A, Pandiella A, et al. Transforming growth factor-beta1 induces collagen synthesis and accumulation via p38 mitogen-activated protein kinase (MAPK) pathway in cultured L(6)E(9) myoblasts. FEBS Lett. 2002; 513:282–288.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download