Abstract
Background
Apart from clinical outcomes, the "real-world" outcomes of intermittent short-course cyclosporine treatment remain poorly documented.
Objective
To
evaluate various outcomes of short-course cyclosporine treatment for severe psoriasis; and to describe dermatologists' use of the Rule of Tens.
Methods
A 12-week pharmacoepidemiological study; 112 evaluable patients recruited by 43 dermatologists.
Results
The mean initial cyclosporine dose was 2.88±0.74 mg/kg/day. At 12 weeks, 64.3% of patients were continued beyond the study period at mean dose of 2.51±0.91 mg/kg/day. Percent body surface affected, Psoriasis Area Severity Index score, and patient and physician rating of psoriasis severity decreased significantly, while quality of life (QoL) improved significantly. Median patient satisfaction at 12 weeks was 85 (0~100 scale). Patient-reported non-adherence was 43.9% and 56.1%, respectively at both the time points (p=0.18). In modeling on logarithmized outcomes variables, living along was consistently the single most important (negative) determinant of therapeutic and patient outcomes. Safety and tolerance parameters were similar to the ones reported in the literature. Only 7.3% of physicians correctly identified the measures included in the Rule of Tens and the Rule's criterion for inferring severe psoriasis.
Conclusion
With adequate monitoring and patient adherence, cyclosporine treatment reduces the severity of severe psoriasis, improves QoL, and is appropriately tolerated; leading to high patient satisfaction. Social support is a key determinant of therapeutic and patient outcomes and patients living along may require clinical attention. The relevance of the Rule of Tens was not evident.
Psoriasis is a systemic immune-mediated chronic skin disease characterized by raised, well-demarcated, erythematous oval plaques with adherent silvery scales frequently associated with arthritis1,2. The prevalence is consistently quoted to be around 2.0%2. Psoriasis is considered severe if more than 10% of a patient's body surface is affected3,4 or if a patient scores more than 10 on the Psoriasis Area Severity Index4. However, there is evidence that this may not adequately reflect the clinical extent of disease5. Psoriasis has profound emotional, social, and physical impact on patients' quality of life5-11. Furthermore, stigmatization may be significant7,11-13. Clinicians' assessment of severity of disease often does not match with their patients' evaluation. Overall, these various factors have an impact on patients' adherence to treatment, while also affecting the treatment outcomes in general14-17. Finlay proposed the Rule of Tens in which a score exceeding 10 on the Body Surface Area (BSA), Psoriasis Area Severity Index (PASI), and/or the Dermatology Life Quality Index constitutes severe psoriasis18.
Intermittent short courses of about 12 weeks of treatment with cyclosporine, in combination with careful monitoring of renal function and blood pressure, is one of the standards of care in the treatment of severe psoriasis3,6,7,19-22. Cyclosporine has been shown to rapidly achieve control of psoriasis, induce remission, and improve quality of life22-29. However, "real-world" outcomes are affected by a number of factors, many of which remain to be poorly understood. The PRACTICE study was a pharmacoepidemiological study examining, under "real world" conditions, cyclosporine treatment patterns and clinical, quality of life (including patient satisfaction), adherence, and safety outcomes of 12-weeks of cyclosporine treatment, initiated as per the prescribing physician's best clinical judgment, in an evaluable sample of 112 patients with severe psoriasis recruited by 43 dermatologists in Belgium. The study also aimed to describe physicians' use of the Rule of Tens and their assessment of the Rule's usefulness and consistency with clinical impressions.
PRACTICE was designed as a prospective, observational, open-label, multi-site pharmacoepidemiological study with two fixed data points: baseline (initiation of cyclosporine treatment) and follow-up at 12 weeks. Interim visits were optional at the treating physician's discretion. The study was approved by the Ethical Committee of University Hospital of Antwerp (Antwerp, Belgium).
Subjects eligible for participation in the study were adult males and females with severe psoriasis being treated with commercially available cyclosporine microemulsion (Neoral®, Novartis, Basel, Switzerland) in accordance with the approved indication approved in Belgium. All subjects provided written informed consent to participate in the study. Excluded were patients previously treated with cyclosporine, patients with renal insufficiency and/or uncontrolled hypertension, patients receiving concomitant ultraviolet B or PUVA phototherapy, or patients with a medical condition which in the investigator's opinion precluded participation in the study.
A total of 167 patients were enrolled by 57 dermatologists. Of these, 112 subjects treated by 43 physicians completed the study and constituted the evaluable sample. Reasons for attrition included loss to follow-up (n=40), adverse events (n=9), patient withdrawal (n=2), patient relocation (n=2), treatment termination because of satisfactory effect (n=1), and unknown reason (n=1). There were no statistically significant differences on key baseline characteristics between the enrollment and evaluable samples, except that there were proportionately more patients with psoriatic arthritis in the evaluable sample (see also Statistical Analysis section).
This being an observational study, only patient data as available from routine clinical practice were recorded and there were no mandatory assessments or tests. Baseline data included: sociodemographics, history of psoriasis, prior treatments for (severe) psoriasis, current psoriasis status (% of Body Surface Area affected [%BSA], PASI), patient-reported quality of life (Dermatology Life Quality Index [DLQI]), physician and patient rating of psoriasis (0~5), serum creatinine, blood pressure, cyclosporine treatment (reason and dose), comorbidities, concomitant medications, and adherence (Basel Assessment of Adherence Scale [BAAS]; patient and physician perceptions Visual Analog Scale [VAS]). At the 12-week follow-up point, data collected included: current psoriasis status (BSA, PASI), quality of life (DLQI), physician and patient rating of psoriasis (0~5), serum creatinine, blood pressure, cyclosporine treatment, concomitant medications, adherence (BAAS and patient and physician VAS), patient satisfaction (VAS), and safety and tolerance. Data collected at optional interim visits included concomitant treatments, cyclosporine treatment, serum creatinine, blood pressure, and safety and tolerance. Because of high intercorrelations among these variables, a composite nonadherence index was calculated from items 1 through 3 of the BAAS and the inverse of the patient VAS self-rating of adherence; yielding an index from 0 (perfect adherence) to 5 (high nonadherence). The study's complete data model is available from the corresponding author.
Data were analyzed using the SPSS ver. 15 (SPSS Inc., Chicago, IL, USA) and SAS (SAS Institute, Cary, NC, USA) statistical programs. In addition to routine descriptive statistics, changes from baseline to follow-up in clinical, DLQI, and adherence outcomes were assessed using the Sign test for dependent samples and proportional odds modeling using Generalized Estimating Equations controlling for psoriatic arthritis. Changes in the proportions of nonadherent patients were evaluated by means of the test for dependent proportions. Random-Intercepts Modeling using Restricted Likelihood Estimation controlling for psoriatic arthritis status was used to model outcomes and assess changes in serum creatinine and blood pressure. Blood pressure control status was examined using McNemar testing. The study was sufficiently powered to detect differences in the primary outcome variables.
The sample ranged in age from 20 to 85 years (mean: 45.0±13.8 years), mostly male (62.1%), with 12th grade or higher education (85.7%), employed (74.3%), and predominantly Caucasian (97.0%). At the start of the study, the majority of patients lived at home with spouse/partner (73.3%) or relatives (7.6%), but 19.1% lived alone. Table 1 summarizes patients' psoriasis history and psoriasis status at enrollment. Mean age at which psoriasis was diagnosed was 34.0±18.1 years (range 1~82); 42.8% had a family member with severe psoriasis. In terms of current disease, 70.7% of patients had the plaque type of psoriasis, on average 8.7±4.2 lesions, mainly on the extremities. Thirteen patients (11.6%) had been diagnosed with psoriatic arthritis. The 93 (83.0%) patients with flares in the six months prior to enrollment had experienced 2.25±1.91 such episodes. The most common prior treatments were vitamin D analogs (72.3%) and PUVA phototherapy (62.5%), though treatment rates were lower at enrollment. On average, patients' treatment regimens had been changed 2.01±1.77 times prior to initiation of cyclosporine treatment.
The reasons for starting patients on cyclosporine included lack of disease control (67.9%), intolerance (9.8%), noncompliance (2.7%), and contra-indication (1.8%), in addition to 3.6% for other nonspecified reasons (14.3% not reported). Initial dosing ranged from 0.50 to 4.38 mg/kg/day (mean 2.88±0.74). At 12-week follow-up, 64.3% of patients were continued on cyclosporine beyond the study period at a mean dose of 2.51±0.91 mg/kg/day, a statistically significant reduction in dose (p=0.005).
Only 7.3% of physicians correctly identified the measures included in the Rule of Tens and the Rule's criterion for inferring severe psoriasis. Mean ratings of the usefulness of the Rule of Tens was 31.1±29.5 and mean ratings of the consistency of the Rule with clinical impressions was 49.6±33.7 (0~100 scale).
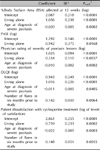
As shown in Table 2, all clinical outcomes (%BSA, PASI, patient and physician ratings of severity of psoriasis) decreased significantly from baseline to follow-up both without and with adjustment for psoriatic arthritis status (all p<0.0001). The quality of life (QOL) scores (DLQI) improved significantly (p<0.0001) and the median patient satisfaction at follow-up was 85 on a 100-point scale (mean 76.0±24.4). Patients and physicians perceptions of patients' adherence were higher at follow-up than at baseline (p ranging from 0.0395 to <0.0001). The percentage of patients reporting nonadherence behavior as per the BAAS increased nominally from 43.9% to 56.1%, but this rise was not statistically significant; and neither was the change observed in the composite nonadherence index.
Modeling on logarithmized outcome variables revealed that the 19.1% of patients living alone tended to have a higher %BSA and PASI score at follow-up, yet %BSA declined in line with how recently the diagnosis of severe psoriasis was made. Physician ratings of severity of lesions tended to be higher for patients living alone, but were attenuated by how recently the diagnosis of severe psoriasis had been made. Patients' QOL (DLQI) tended to be lower if patients lived alone. It also decreased with each flare experienced in the six months prior to the start of cyclosporine therapy. It was mitigated by how recently the severe psoriasis diagnosis had been made. Patient satisfaction was found to be affected negatively among patients living alone, but was influenced positively by a more recent diagnosis of psoriasis and less flares having been experienced in the six months prior to the start of cyclosporine treatment (Table 3).
Adverse events were reported in 36.0% of patients. Most frequently reported were neurological symptoms (headache, paresthesia, and tremor) and gastrointestinal disorders. Fourteen percent of patients discontinued the treatment due to adverse events. Mean serum creatinine levels significantly increased by 0.05 mg/dl over the 12-week treatment period (p=0.0001; Table 4). Only two patients experienced an increase in serum creatinine >30.0% of baseline in two subsequent measurements (threshold of maximal increase as outlined in the scientific leaflet). There was a mean 4 mmHg increase in systolic (p=0.003) and 3 mmHg increase in diastolic (p=0.003) blood pressure from baseline to the end of the study; about 20% of the patients became hypertensive during the course of the study (p<0.02)
The principal findings of this study on "real-world" short-course (12 weeks) treatment of severe psoriasis with cyclosporine is that this agent reduces objective dermatological parameters (%BSA and PASI), provides effective control of the disease, improves quality of life, and is associated with high patient satisfaction. Safety outcomes were in line with those reported in the literature.
The %BSA affected declined by 70.0% in mean (from 23.4% to 7.0%) and by 74.2% in median (from 15.5% to 4.0%). The PASI scores decreased by 67.8% in mean (from 15.88 to 5.11) and by 78.4% in median (from 13.9 to 3.0). Thus, both the psoriasis parameters were reduced from the severe to the non-severe range after 12 weeks of cyclosporine treatment. These results are consistent with the efficacy of cyclosporine shown in randomized controlled trials on psoriasis1,4,20-22. Physicians and patients concurred in their evaluation of clinical improvement. Patient severity ratings lessened by 65.9% in mean (from 4.02 to 1.37) and by 75.0% in median (from 4.0 to 1.0), and physicians reduced their ratings of lesion severity by 61.4% in mean (from 3.60 to 1.39) and by 75.0% in median (from 4.0 to 1.0).
It is not surprising to note that patients tended to be very satisfied with their cyclosporine treatment and rated their quality of life as having improved by 70.2% in mean (from 12.13 to 3.61) and by 83.4% in median. Patient satisfaction with cyclosporine treatment was quite high (median of 85 on 0~100 scale), which is known to be associated with better clinical outcomes16,30.
These strong results are despite the fact that nonadherence was a clinical issue in this sample, as it is known to be in dermatology in general15,16. Whether cyclosporine therapy increases patients' adherence is not clear from our study. Subjective patients' and physicians' adherence ratings suggest that there may have been an improvement. However, actual nonadherence behavior was highly prevalent (43.9% at baseline and 56.1% at follow-up).
The single most important determinant of therapeutic and patient outcomes, as revealed in the multilevel models, was the negative impact of living alone (which 19.1% of patients did). Generally, patients who live with a spouse/partner or relatives have the benefit of an additional caregiver to complement their own self-care, something that those living alone do not have. In addition, living alone may create a sense of social isolation (or lack of social support) that colors perceptions of quality of life and satisfaction with treatment10. A more recent diagnosis of severe psoriasis was associated with better %BSA, QOL, and patient satisfaction outcomes. In addition, patients who had suffered more flares prior to the initiation of cyclosporine treatment reported lower QOL and treatment satisfaction. This observation points at the potential role of chronicity of illness and suffering among patients with severe psoriasis.
Safety and tolerance parameters were similar to those reported in the literature. Patients on cyclosporine need to be carefully monitored for nephrotoxicity, hypertension and other side effects22. Current treatment guidelines stipulate reduction of cyclosporine dose if serum creatinine increases by 30.0% above the baseline value. However, any changes in renal function are usually functional and are normally reversed by cessation of cyclosporine therapy4. If blood pressure increases beyond the upper limit of normal, cyclosporine dose should be reduced by 25.0~50.0% and/or an appropriate antihypertensive agent should be started4.
Physicians' use and perceived value of the Rule of Tens, which aims to integrate dermatological parameters with patient perspectives to define severe psoriasis, was limited. The relevance and validity of the Rule of Tens requires further investigation.
Despite this study being limited to one country, only one treatment cycle, and not being population-based but sampled by convenience, it confirms that short-course (12 weeks) cyclosporine treatment, with appropriate safety monitoring of renal and blood pressure parameters, is highly effective in adherent patients and is associated with high patient satisfaction.
Figures and Tables
Table 2
Changes in treatment outcomes

SD: standard deviation, Mdn: median, %BSA: % of Body Surface Area affected, PASI: Psoriasis Area Severity Index, DLQI: Dermatology Life Quality Index, VAS: Visual Analog Scale, BAAS: Basel Assessment of Adherence Scale. *Significance level for the Sign test. †Significance level for Generalized Estimating Equations analysis, controlling for psoriatic arthritis status. ‡Significance level for the test for dependent proportions.
ACKNOWLEDGMENT
This study was sponsored by Novartis Pharma. We thank patients as well as investigators and staff for their participation in the study. We also thank Brian Yee, Erin Arizmendi, and Mei So for editorial, proofreading, and administrative assistance.
Poster presentation at the 4th Annual Meeting of the Belgian Association of Pharmaceutical Physicians, Brussels, Belgium (18 March 2010).
References
2. Christophers E. Psoriasis--epidemiology and clinical spectrum. Clin Exp Dermatol. 2001. 26:314–320.
3. Krueger GG, Feldman SR, Camisa C, Duvic M, Elder JT, Gottlieb AB, et al. Two considerations for patients with psoriasis and their clinicians: what defines mild, moderate, and severe psoriasis? What constitutes a clinically significant improvement when treating psoriasis? J Am Acad Dermatol. 2000. 43:281–285.
4. Griffiths CE, Dubertret L, Ellis CN, Finlay AY, Finzi AF, Ho VC, et al. Ciclosporin in psoriasis clinical practice: an international consensus statement. Br J Dermatol. 2004. 150:Suppl 67. 11–23.

5. Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol. 1999. 41:401–407.

6. Kirby B, Fortune DG, Bhushan M, Chalmers RJ, Griffiths CE. The Salford Psoriasis Index: an holistic measure of psoriasis severity. Br J Dermatol. 2000. 142:728–732.

7. Finlay AY. Psoriasis from the patient's point of view. Arch Dermatol. 2001. 137:352–353.
8. Krueger G, Koo J, Lebwohl M, Menter A, Stern RS, Rolstad T. The impact of psoriasis on quality of life: results of a 1998 National Psoriasis Foundation patient-membership survey. Arch Dermatol. 2001. 137:280–284.
9. Zachariae R, Zachariae H, Blomqvist K, Davidsson S, Molin L, Mørk C, et al. Quality of life in 6497 Nordic patients with psoriasis. Br J Dermatol. 2002. 146:1006–1016.

10. Seidler EM, Kimball AB. Socioeconomic disability in psoriasis. Br J Dermatol. 2009. 161:1410–1412.

12. Richards HL, Fortune DG, Griffiths CE, Main CJ. The contribution of perceptions of stigmatisation to disability in patients with psoriasis. J Psychosom Res. 2001. 50:11–15.

13. Vardy D, Besser A, Amir M, Gesthalter B, Biton A, Buskila D. Experiences of stigmatization play a role in mediating the impact of disease severity on quality of life in psoriasis patients. Br J Dermatol. 2002. 147:736–742.

14. Richards HL, Fortune DG, Griffiths CE. Adherence to treatment in patients with psoriasis. J Eur Acad Dermatol Venereol. 2006. 20:370–379.

15. Feldman SR, Horn EJ, Balkrishnan R, Basra MK, Finlay AY, McCoy D, et al. Psoriasis: improving adherence to topical therapy. J Am Acad Dermatol. 2008. 59:1009–1016.

16. Ali SM, Brodell RT, Balkrishnan R, Feldman SR. Poor adherence to treatments: a fundamental principle of dermatology. Arch Dermatol. 2007. 143:912–915.
17. Ho VC. The use of ciclosporin in psoriasis: a clinical review. Br J Dermatol. 2004. 150:Suppl 67. 1–10.

20. Madan V, Griffiths CE. Systemic ciclosporin and tacrolimus in dermatology. Dermatol Ther. 2007. 20:239–250.

21. Warren RB, Griffiths CE. Systemic therapies for psoriasis: methotrexate, retinoids, and cyclosporine. Clin Dermatol. 2008. 26:438–447.

22. Rosmarin DM, Lebwohl M, Elewski BE, Gottlieb AB. National Psoriasis Foundation. Cyclosporine and psoriasis: 2008 National Psoriasis Foundation Consensus Conference. J Am Acad Dermatol. 2010. 62:838–853.

23. Ho VC, Griffiths CE, Albrecht G, Vanaclocha F, León-Dorantes G, Atakan N, et al. The PISCES Study Group. Intermittent short courses of cyclosporin (Neoral(R)) for psoriasis unresponsive to topical therapy: a 1-year multicentre, randomized study. Br J Dermatol. 1999. 141:283–291.

24. Ho VC, Griffiths CE, Berth-Jones J, Papp KA, Vanaclocha F, Dauden E, et al. Intermittent short courses of cyclosporine microemulsion for the long-term management of psoriasis: a 2-year cohort study. J Am Acad Dermatol. 2001. 44:643–651.

25. Touw CR, Hakkaart-Van Roijen L, Verboom P, Paul C, Rutten FF, Finlay AY. Quality of life and clinical outcome in psoriasis patients using intermittent cyclosporin. Br J Dermatol. 2001. 144:967–972.

26. Heydendael VM, Spuls PI, Opmeer BC, de Borgie CA, Reitsma JB, Goldschmidt WF, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003. 349:658–665.

27. Salek MS, Finlay AY, Lewis JJ, Sumner MI. Quality of life improvement in treatment of psoriasis with intermittent short course cyclosporin (Neoral). Qual Life Res. 2004. 13:91–95.

28. Chaidemenos GC, Mourellou O, Avgoustinaki N, Papakonstantinou M, Karakatsanis G, Katsambas A. Intermittent vs. continuous 1-year cyclosporin use in chronic plaque psoriasis. J Eur Acad Dermatol Venereol. 2007. 21:1203–1208.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download