Abstract
Onychomycosis is usually caused by dermatophytes, but some nondermatophytic molds and yeasts are also associated with invasion of nails. The genus Chaetomium is a dematiaceous nondermatophytic mold found in soil and plant debris as a saprophytic fungus. We report the first Korean case of onychomycosis caused by Chaetomium globosum in a 35-year-old male. The patient showed brownish-yellow discoloration and subungual hyperkeratosis on the right toenails (1st and 5th) and left toenails (1st and 4th). Direct microscopic examination of scraping on the potassium hydroxide preparation revealed septate hyphae and repeated cultures on Sabouraud's dextrose agar (SDA) without cycloheximide slants showed the same fast-growing colonies, which were initially velvety white then turned to dark gray to brown. However, there was no growth of colony on SDA with cycloheximide slants. Brown-colored septated hyphae, perithecia and ascospores were shown in the slide culture. The DNA sequence of internal transcribed spacer region of the clinical sample was a 100% match to that of C. globosum strain ATCC 6205 (GenBank accession number EF524036.1). We confirmed C. globosum by KOH mount, colony, and light microscopic morphology and DNA sequence analysis. The patient was treated with 250 mg oral terbinafine daily and topical amorolfine 5% nail lacquer for 3 months.
Onychomycosis is caused mainly by dermatophytes but occasionally by nondermatophytic fungi including Scopulariopsis brevicaulis, Aspergillus species (spp.), Fusarium spp., Acremonium spp., and Chaetomium spp. which have often been considered as saprophytic or opportunistic fungi1. So far such molds have been regarded as saprophytic or opportunistic fungi and thus have been ignored. Recently, as a consequence of the increase in the number of cases of immune suppression and environmental changes, more attention has been given to this wide, but generally not pathogenic group of fungi2. This apparent emergence might be an artifact of improved diagnostic techniques and increased awareness that these fungi are potential etiologic agents3.
The genus Chaetomium, which belongs to ascomycetes, is a dematiaceous mold found worldwide in soil and plant debris as a saprophyte. Chaetomium species are rarely involved in human infection, but have been reported to cause subcutaneous phaeohyphomycosis and systemic infections in immunocompromised patients as well as onychomycosis in healthy subjects4. There have been only four cases of onychomycosis caused by Chaetomium globosum, which is the most frequently isolated species among Chaetomium spp.4-10.
Here, we report the first case in Korea of onychomycosis caused by C. globosum. The identification of the causative fungus was confirmed by clinical findings, repeated fungal isolation, light microscopy and sequencing analysis of the internal transcribed spacer (ITS) region in ribosomal RNA genes.
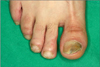
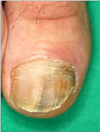
A 35-year-old male presented with a 2-year history of brownish-yellow discoloration and subungual hyperkeratosis on the right toenails (1st and 5th) and left toenails (1st and 4th) (Fig. 1, 2). The patient was otherwise in good health and he denied nail trauma or dystrophic nail abnormalities prior to the onset of the present lesions. There was no history of other diseases except for toenail dystrophy. On the visit, laboratory studies including a complete blood cell count with differentials, liver and renal function test, venereal disease research laboratory, urinalysis, stool examination, hepatitis viral test, human immunodeficiency virus test, chest X-ray, and electrocardiogram were all within normal limits or negative.
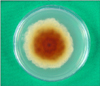
In mycological examination, septated hyphae were observed in 20% KOH preparation from the toenail lesions. Nail specimens were cultured on two Sabouraud's dextrose agar (SDA) without cycloheximide slants at 25℃ for a week to yield several identical colonies. However, there was no growth of colony on SDA with cycloheximide slants. These rapid growing colonies were initially velvety white then turned to dark gray to brown (Fig. 3). The reverse surface of the colonies revealed an orange-tan color. Subcultures on agar plates exhibited the same results (Fig. 4, 5). When the slide cultures of fungal colonies were stained with lactophenol cotton blue, the morphological characteristics such as brown-colored septated hyphae, perithecia and ascospores were observed by light microscopy (Fig. 6). Perithecia were large and dark brown to black color, revealing a globose to flask shape with unbranched, hair-like filamentous appendages on their surface. Perithecia demonstrated ostioles and contained asci and single-celled ascospores which were olive brown and lemon-shaped (Fig. 7, 8).
The ITS region of ribosomal RNA genes was amplified using DNA of the fungal colonies cultured from nail specimens. For molecular biologic analysis, DNA was extracted from the cultured colonies and the base sequence of ITS was identified. Subsequently, it was compared to the base sequence of C. globosum strain ATCC 6205 (GenBank accession number: EF524036.1), which was stored in GenBank, using the Blast program. The result was 100% matched (Fig. 9).
Based on the KOH mount, mycological finding, light microscopy and sequence analysis of the ITS region, the isolate was finally identified as C. globosum. The patient was treated with 250 mg oral terbinafine daily and topical amorolfine 5% nail lacquer for 3 months.
Onychomycoses comprising 50% of all onychopathies are caused mainly by dermatophytes and less frequently by nondermatophytic molds and yeasts1. Onychomycosis caused by nondermatophytic molds comprises 1.45 to 17.6% of the total cases, and Aspergillus spp., Scopulariopsis spp., Fusarium spp., Acremonium spp., and Chaetomium spp. may be the causes2,11-13.
Since the first definition of the genus Chaetomium was made by Kunce in 1,817, more than 105 species have been identifined4. They are usually found in association with cellulose-containing substrates such as wood, straw and paper9. Among the 105 species of genus Chaetomium, C. globosum, C. atrobrunneum, C. strumarium, C. perlucidum, and C. funicolum cause infections in the human body. C. globosum is most frequently isolated from patients with superficial mycoses such as onychomycosis and cutaneous phaeohyphomycosis. As far as we know, there are four reported cases of onychomycosis caused by C. globosum, which clearly demonstrated that Chaetomium was the cause of extensive onychomycosis4,7-10. This is the first report of Chaetomium infection of the nail in a patient in Korea.
As nondermatophytic molds including C. globosum are saprophytes, it is not feasible to distinguish the causative agents from contaminants when these molds are isolated from the nail specimens14. Therefore, it should be checked if the characteristic hyphae or spores are present in KOH preparation, if identical colonies are obtained from specimens, and if the same causative mold is identified from repeated cultures15. In this case, fungal elements were found by microscopy, and the same fungal species were isolated from the repetitive cultures of toenail specimens.
The identification of C. globosum had been based on the morphological features of the colonies. Lately, however, sequencing analysis of the ITS region of ribosomal DNA has been considered necessary for definite identification among the genus Chaetomium16,17. Morphological features of C. globosum include fast-growing dark gray colonies, peritheca with hairy filamentous appendages, and ostioles at the bottom of peritheca containing asci and ascospores. Ascospores are lemon shaped olive-brown single cells. Our case revealed not only the typical morphological features of C. globosum but also a perfect homology to the ITS sequence of C. globosum ATCC 6205, confirming the identification of C. globosum.
A comparison made between this case and the other reported cases (Table 1) showed that all cases occurred in adults in their 20s and older and lasted 2 months to 4 years7-10. In addition, they occurred more in men's toenails. All five patients showed brownish discoloration without periungual inflammation. In Aspiroz et al.9 and Latha et al.10, the patients had trauma history. In this case, however, no patient had a history of trauma.
It is known that onychomycosis caused by nondermatophytic mold does not respond well to common treatment18. Guarro et al.19 tested 23 strains of Chaetomium species from patients and environments against 6 antifungal agents in vitro (5-fluorocytosine, fluconazole, amphotericin B, itraconazole, ketoconazole, and miconazole). All 23 strains were resistant to the first two drugs, and none of the other antifungal agents demonstrated fungicidal activity to the organisms. In Aspiroz et al.9, oral terbinafine was administered to the patient with C. globosum onychomycosis for 3 months, and the patient was completely cured. Similar studies about other nondermatophytic molds show that the treatment with terbinafine and itraconazole can be considered effective. Likewise, in this case, oral terbinafine was administered 250 mg a day in addition to the local application of amorolfine 5% nail lacquer. As a result, the patient was completely cured clinically and mycologically as well.
In conclusion, we report the first Korean case of onychomycosis caused by C. globosum in an immunocompetent patient which was identified by morphological features as well as molecular analysis. If C. globosum is isolated from onychomycosis patients, one should not disregard this as a contaminant but a causative agent requiring further mycological studies.
Figures and Tables
Fig. 3
Multiple, dark grey to brown colonies with aerial mycelium on Sabouraud's dextrose agar slants after incubation at 25℃ for 1 week.
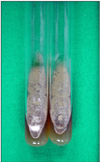
Fig. 4
A rapid growing, dark grey to brown colony with aerial mycelium on Sabouraud's dextrose agar plate after incubation at 25℃ for 1 week.
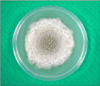
Fig. 6
Large, dark brown to black, flask shaped perithecia with hair-like filamentous appendages (Lactophenol cotton blue stain, ×400).
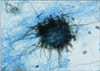
Fig. 7
Close-up view of perithecium which demonstrates ostioles and contains asci and single-celled ascospores (Lactophenol cotton blue stain, ×200).
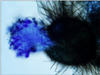
Fig. 8
Brown-colored septated hyphae and lemon-shaped ascospores (Lactophenol cotton blue stain, ×400).
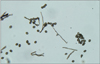
Fig. 9
Alignment of internal transcribed spacer (ITS) sequences of the sample from the patient and Chaetomium globosum strain ATCC 6205 (GenBank accession number EF524036.1). The sequences of ITS for the clinical sample was a 100% match to that of C. globosum strain ATCC 6205 (GenBank accession number EF524036.1).

References
1. Verma S, Heffernan MP. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Superficial fungal infection: dermatophytosis, onychomycosis, tinea nigra, piedra. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1807–1821.
2. Gianni C, Cerri A, Crosti C. Non-dermatophytic onychomycosis. An understimated entity? A study of 51 cases. Mycoses. 2000. 43:29–33.

3. Gupta AK, Ryder JE, Baran R, Summerbell RC. Non-dermatophyte onychomycosis. Dermatol Clin. 2003. 21:257–268.

4. Falcón CS, Falcón Ma del MS, Ceballos JD, Florencio VD, Erchiga VC, Ortega SS. Onychomycosis by Chaetomium spp. Mycoses. 2009. 52:77–79.
5. Rippon JW. Medical mycology: the pathogenic fungi and the pathogenic actinomycetes. 1988. 3rd ed. Philadelphia: WB Saunders;213.
6. Naidu J, Singh SM, Pouranik M. Onychomycosis caused by Chaetomium globosum Kunze. Mycopathologia. 1991. 113:31–34.

7. Stiller MJ, Rosenthal S, Summerbell RC, Pollack J, Chan A. Onychomycosis of the toenails caused by Chaetomium globosum. J Am Acad Dermatol. 1992. 26:775–776.

8. Hattori N, Adachi M, Kaneko T, Shimozuma M, Ichinohe M, Iozumi K. Case report. Onychomycosis due to Chaetomium globosum successfully treated with itraconazole. Mycoses. 2000. 43:89–92.

9. Aspiroz C, Gené J, Rezusta A, Charlez L, Summerbell RC. First Spanish case of onychomycosis caused by Chaetomium globosum. Med Mycol. 2007. 45:279–282.

10. Latha R, Sasikala R, Muruganandam N, Shiva Prakash MR. Onychomycosis due to ascomycete Chaetomium globosum: a case report. Indian J Pathol Microbiol. 2010. 53:566–567.

11. Gianni C, Romano C. Clinical and histological aspects of toenail onychomycosis caused by Aspergillus spp.: 34 cases treated with weekly intermittent terbinafine. Dermatology. 2004. 209:104–110.

12. Tosti A, Piraccini BM, Lorenzi S. Onychomycosis caused by nondermatophytic molds: clinical features and response to treatment of 59 cases. J Am Acad Dermatol. 2000. 42:217–224.

13. Summerbell RC, Kane J, Krajden S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses. 1989. 32:609–619.

14. Arrese JE, Piérard-Franchimont C, Piérard GE. Unusual mould infection of the human stratum corneum. J Med Vet Mycol. 1997. 35:225–227.

16. Yu J, Yang S, Zhao Y, Li R. A case of subcutaneous phaeoPhyphomycosis caused by Chaetomium globosum and the sequences analysis of C. globosum. Med Mycol. 2006. 44:541–545.

17. Tullio V, Banche G, Allizond V, Roana J, Mandras N, Scalas D, et al. Non-dermatophyte moulds as skin and nail foot mycosis agents: Phoma herbarum, Chaetomium globosum and Microascus cinereus. Fungal Biol. 2010. 114:345–349.

18. Nolting S, Brautigam M, Weidinger G. Terbinafine in onychomycosis with involvement by non-dermatophytic fungi. Br J Dermatol. 1994. 130:Suppl 43. 16–21.

19. Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis. 1995. 14:613–618.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download