Abstract
Trastuzumab (Herceptin), a humanized monoclonal antibody, is a cancer drug developed to target the human epidermal receptor (HER) 2, which is overexpressed in some cancer cells. Cutaneous side effects, such as folliculitis, xerosis, and alopecia have not been reported with therapies targeting HER2, in spite of the frequent observances of such with the therapies targeting the epidermal growth factor receptor. We experienced a patient in whom psoriasis was triggered by the trastuzumab treatment for breast cancer. She was a 57-year-old woman with erythematous and scaly plaques occurring a few months after starting trastuzumab, with repeated aggravation after the re-administration of trastuzumab for the breast cancer. Histologic examination showed the typical features of psoriasis with parakeratosis, epidermal hyperplasia, elongation of the rete ridges, and a lymphocytic and polymorphonuclear cell infiltrate in the dermis. To the best of our knowledge, this is the first report of psoriasis triggered by trastuzumab treatment for breast cancer.
There are several important tumor subtypes in the breast cancers, each with a different natural history and requiring a different treatment. The overexpression of human epidermal receptor (HER) 2 is observed in 20 to 25% of the breast cancer cases and defines one of the unique subtypes which is associated with a poor prognosis1. Thus, an anti-HER2 agent, such as trastuzumab, is indicated for the adjuvant treatment of the early HER2-positive breast cancers and the treatment of metastatic HER2-positive breast cancer. Trastuzumab and other anti-HER2 agents are expected to improve the long-term survival2.
Therapeutic agents targeting the HER family are being used frequently for a variety of solid tumors. HER inhibitors have numerous cutaneous side effects, especially the agents that inhibit the epidermal growth factor receptor (EGFR) or HER1. However, the inhibitors of HER2 are not associated with a specific skin toxicity2-4.
We present a case of psoriasis in a woman being treated with trastuzumab, a selective HER2 inhibitor. Psoriasis has not previously been reported as a cutaneous side effect of the trastuzumab therapy.
A 57-year-old woman was referred to our dermatologic outpatient clinic for the evaluation and treatment of psoriatic skin lesions after the trastuzumab treatment for breast cancer. She had been diagnosed with invasive ductal carcinoma of the left breast in August of 2009. She had been in relatively good health without any medical problems, and she had no personal or family history of psoriasis.
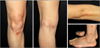
After the surgical removal of the left breast, she was treated with trastuzumab every 3 weeks for the HER2+ breast cancer from November 2009 to the time of our examination. A few months after the treatment began, she developed erythematous and scaly plaques on her extremities (Fig. 1). She was treated with a combination of the ultraviolet B phototherapy and a topical steroid, and her skin lesions improved. However, when trastuzumab was re-administrated, the skin lesions were exacerbated without other possible aggravating factors.
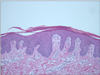
A skin biopsy from a typical lesion of the left leg showed the typical features of psoriasis, with parakeratosis, epidermal hyperplasia, elongation of the rete ridges, dilated capillaries in the dermal papillae, and a lymphocytic and polymorphonuclear cell infiltrate in the dermis (Fig. 2). Because her skin condition responded well to the treatment with a topical steroid and phototherapy, the discontinuation of trastuzumab was not required.
We report the new onset of psoriasis in a patient who was receiving an anti-HER2 agent for HER2+ breast cancer. The patient exhibited no clinical evidence of psoriasis or psoriasiform skin lesions either before or at the time of the initiation of the treatment with the anti-HER2 agent. The clinical features and histologic findings showed the typical features of psoriasis. Skin lesions were repeatedly aggravated by the trastuzumab treatment for breast cancer. However, trastuzumab was not discontinued because of its highly beneficial effect for the cancer treatment, and the skin condition responded well to an alternative treatment.
The HER family consists of four structurally-related receptor tyrosine kinases, including the EGFR, HER2, HER3, and HER4. These factors mediate cell growth, differentiation, and survival via signal transduction pathways4. EGFR and HER2, in particular, play key roles in the tumorigenic process of epithelial cancers and are being used frequently for the target-based treatments of various solid tumors, such as cancers of the lung, colon, and breast3,5,6.
EGFR inhibitors have numerous cutaneous side effects, including the characteristic papulopustular folliculitis, xerosis, pyogenic granuloma, paronychia, and scarring and nonscarring alopecia3,4. However, the anti-HER2 agents such as trastuzumab and pertuzumab have been reported only with the non-specific skin reactions and primarily the infusion reactions similar to those seen with other monoclonal antibodies2-4. These differences in the dermatologic toxicity are associated with the dimerization status of the HER family members in the skin. The HER signaling network functions can only occur after the formation of receptor dimers2. EGFR homodimers are the predominant isoform in human keratinocytes, while few or no HER2 heterodimers are found3. Therefore, this discrepancy may be attributed to an absence of a specific skin reaction to the HER2 inhibitors.
Trastuzumab-induced psoriasis has not previously been reported, and the conclusive knowledge regarding the relationship between psoriasis and the role of HER2 is lacking. In our case, the following strongly supported a diagnosis of the trastuzumab-induced psoriasis: 1) no personal or family history of psoriasis; 2) no other triggering factors known to induce psoriasis, such as co-medication, smoking, or infection; and 3) aggravation of psoriasis after the re-administration of trastuzumab.
Although the role of HER2 in the skin remains unclear, some evidence suggests that HER2 plays an active role in the keratinocyte differentiation7. In addition, De potter et al. reported that some HER ligands activate the signaling transduction pathway of the HER2 heterodimer rather than the EGFR homodimer in the subpopulations of differentiating keratinocytes7,8. Thus, certain HER2 inhibitors, regardless of EGFR, can lead to an alteration of the normal epidermal differentiation and turnover. Furthermore, psoriatic skin in which both EGFR and HER2 are extensively expressed may show a different HER dimerization status compared with the normal skin. Identification of such a difference and identification of the relationship with a specific HER modulator will help clarify the situation.
We present a case of psoriasiform eruption triggered by the trastuzumab therapy in a patient with HER2+ breast cancer. The mechanism underlying this observation cannot be readily explained by the currently available data. However, our report takes the first step towards addressing the associations between psoriasis and HER2, and analyzes the molecular mechanism potentially responsible for the skin toxicity observed in patients treated with the HER2-directed therapies.
Figures and Tables
References
1. Chang HR. Trastuzumab-based neoadjuvant therapy in patients with HER2-positive breast cancer. Cancer. 2010. 116:2856–2867.

2. Garnock-Jones KP, Keating GM, Scott LJ. Trastuzumab: a review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. Drugs. 2010. 70:215–239.
3. Laux I, Jain A, Singh S, Agus DB. Epidermal growth factor receptor dimerization status determines skin toxicity to HER-kinase targeted therapies. Br J Cancer. 2006. 94:85–92.

4. Myskowski PL, Halpern AC. Skin reactions to the new biologic anticancer drugs. Curr Opin Support Palliat Care. 2009. 3:294–299.

5. Arteaga C. Targeting HER1/EGFR: a molecular approach to cancer therapy. Semin Oncol. 2003. 30:3 Suppl 7. 3–14.

6. Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000. 19:6550–6565.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download