INTRODUCTION
Vitiligo is characterized by the loss of epidermal melanocytes. Autoimmunity and oxidative stress as well as genetic predisposition have been implicated in the pathogenesis of vitiligo1. In contrast, dermal melanocytosis is characterized by the presence of dermal dendritic cells that resemble melanocytes that migrate from the neural crest to the epidermis2. It includes several benign pigmented lesions like Mongolian spot, nevus of Ota and nevus of Ito which are more common in Asian populations3. The association of these two conditions is rarely reported4,5.
Here we report two cases of vitiligo associated with dermal melanocytosis which persisted after the development of vitiligo.
CASE REPORT
Case 1
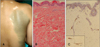
A 20-year-old woman presented with dermal melanocytic nevus and a one-year history of vitiligo. The patient had a large bluish patch on the right upper back that had been present since birth. There was no significant past medical history and no family history of autoimmune disease. The laboratory studies were unremarkable. Physical examination revealed several white patches on the anterior chest and right scapula. They were partially overlapping the preexisting bluish patch with a faint bluish hue (Fig. 1A). Histologically, the overlapping lesion revealed an elongated spindle or dendritic cells containing melanin granules scattered within the dermis. No infiltration of inflammatory cell was observed (Fig. 1B). The S-100 protein immunoperoxidase staining was positive in the dermal dendritic cells, and the lesion was devoid of epidermal melanocytes (Fig. 1C).
Case 2
A 23-year-old man presented with a four-year history of extensive depigmented patches. These lesions overlapped with bluish patches which had been present since the age of three (Fig. 2A, B). There was no significant past medical and familial history. The laboratory tests were within the normal range. A biopsy specimen was obtained from the border of the vitiliginous patch that overlapped the bluish patches. The histopathology showed dermal melanocytes with the loss of epidermal melanocytes (Fig. 2C, D). There was no inflammatory cell infiltrate at the dermalepidermal junction or in the dermis.
DISCUSSION
Cases of vitiligo associated with dermal melanocytosis have been rarely reported. Hamada et al.4 first reported the association of these pigmentary diseases. Luo et al.5 only recently reported a similar case where the nevus of Ota associated with vitiligo occurred in an 11-year-old Chinese boy.
In the overlapping lesions of these cases, the loss of melanocytes was observed only in the epidermal basal layer. The dermal melanocytes from these lesions revealed a similar histological finding with typical cases of dermal melanocytosis. There was no significant infiltrative lymphocytic response in the upper dermis and at the epidermal-dermal junction. Considering the pathogenesis of this finding, the immune response against nevus cells is less likely to have an effect on the epidermal melanocytes. 'Vitiligo melanocytes' are believed to have defects in their ability to scavenge the toxic intermediates of melanin biosynthesis leading to their programmed death6. Also, it has been proposed that the epidermal environment could be involved in the pathogenesis of vitiligo. The altered metabolism of epidermal tetrahydrobiopterin may lead to an inhibition of antioxidant enzymes and melanin synthesis and to an increased production of catecholamines7.
Keratinocytes in vitiliginous lesions also play a role in epidermal melanocyte destruction. The keratinocytes in vitiligo produce pro-inflammatory cytokines including interleukin (IL)-1α, IL-6 and tumor necrosis factor-α, thus provoking the inflammatory process1; Keratinocytes in the vitiligo lesion are defective in producing specific melanocyte growth factors and thus, facilitate melanocyte apoptosis8.
In this context, vitiligo development on the site of persisting dermal melanocytosis means the uncertain mechanism affects epidermal and dermal melanocytes in different manners. It could be based on the difference between the epidermal and the dermal environments, or between the properties of the normal and the ectopic melanocytes. First, increased oxidative stress in the vitiliginous epidermis may induce the loss of epidermal, rather than dermal, melanocytes9. Second, the 'vitiligo melanocyte' is inherently vulnerable to noxious stimuli due to the intrinsic defect in its defense mechanisms1. The 'dermal melanocyte' may have greater resistance to external stress compared to the 'vitiligo melanocyte'. The third piece of supporting evidence involves the diversity in antigen expression. Shin et al.10 reported a case with a congenital giant melanocytic nevus that developed neurotization of the nevus cell combined with vitiliginous changes within the lesion. They hypothesized that this presentation may result from the sharing of common pigment cell antigens between a normal melanocyte and a nevus cell. However, the absence of inflammatory cell infiltrates as observed in the halo nevus, and epidermal melanocytic loss in the current reports raises the possibility of antigen diversity between epidermal and dermal melanocytes.
We could not completely rule out the possibility of an inflammatory reaction against dermal melanocyte resulting in the development of vitiligo in spite of the absence of inflammatory cell infiltration in our cases. However, considering the vitiligo developed at distant sites from the dermal melanocytosis lesion, the possibility of the above-mentioned mechanism is unlikely.
We presented two cases of vitiligo on the pre-existing dermal melanocytosis lesions. Very few similar cases have been reported and the change of dermal melanocytes in this setting has not been observed4. Therefore, it is very interesting to investigate the change of dermal melanocyte and inflammatory cell infiltrates in this unusual situation. The results reported here illustrate that dermal melanocytes are not affected in contrast to the epidermal melanocytes. However, further study is needed to clarify the reason for this finding.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download