Abstract
Background
It is well known that atopic dermatitis (AD) is related to food hypersensitivity, although its prevalence varies among several studies according to age group, severity, country, survey time, and test method.
Objective
To examine the prevalence and status of food hypersensitivity among childhood AD patients in Korea.
Methods
A total of 95 patients were enrolled in the study. The history of food hypersensitivity was collected by interviews. The severity of AD was evaluated by eczema area and severity index (EASI). We took blood samples to measure serum total and food-specific immunoglobulin E (IgE) levels. Based on the histories and serum IgE levels, open oral food challenge (OFC) testing was performed to confirm food hypersensitivity.
Results
Forty-two (44.2%) of the 95 AD patients had histories of food hypersensitivity. They reported that the most common suspicious foods were egg (n=13, 13.7%), pork (n=9, 9.5%) and cow milk (n=8, 8.4%). The mean EASI score was 16.05±9.76. Thirty-nine (41.1%) of the 95 patients showed elevated serum food-specific IgE levels. The specific IgE levels were elevated for egg (n=17, 17.9%), milk (n=12, 12.6%), peanut (n=10, 10.5%) and wheat (n=8, 8.4%). Fifty-one (53.8%) of 95 patients underwent open OFC, and only 7 (13.7%) of these patients showed positive reactions.
Conclusion
The overall prevalence of food hypersensitivity in patients with childhood AD in Korea was 8.3% (7/84). The most common foods causing food hypersensitivity were egg and milk. Among the foods causing hypersensitivity, AD patients in Korea often underestimated peanut, while they overestimated pork.
Atopic dermatitis (AD) is a common skin disorder and affects up to 10% of children ages 3 to 6 years in Korea1. AD is related to other allergic diseases, such as asthma, allergic rhinitis, and food allergy2. Since Schloss3 first described AD in 1915 how AD patients improved by avoiding specific foods, many studies have been conducted to examine the relationship between AD and food hypersensitivity4. Nevertheless, the role of food hypersensitivity in the pathogenesis of AD still remains controversial5.
Food hypersensitivity in AD patients occurs as either immediate immunoglobulin E (IgE)-mediated hypersensitivity or as delayed cell-mediated hypersensitivity. The prevalence of food hypersensitivity in AD patients varies among several studies in terms of age group, severity, country, survey times, and test methods4,6. In Korea, the prevalence of food allergy in AD patients were reported as 9.5% on a cross-sectional questionnaire survey and 18.2% based on open food challenge tests conducted by the dermatology clinic of a certain university hospital7,8. The present study aimed to examine the prevalence and status of food hypersensitivity in patients with childhood AD in Korea.
This study included patients who were diagnosed with AD at the Department of Dermatology, Hallym University, Kangnam Sacred Heart Hospital, Seoul, Korea between August 2009 and December 2010. The diagnosis of AD was based on the diagnostic criteria of Korean AD, modified from criteria developed by Hanifin and Rajka9. A total of 95 subjects (52 males, 43 females) aged 2 to 18 years (mean age, 6.85±4.37 years) were enrolled in the study. The protocol was approved by the Institutional Review Board of Kangnam Sacred Heart Hospital. Informed consent was obtained from each participant and their parents if they are age 18 years and under.
The medical information collected from the patients included the previous history of food hypersensitivity, causative food allergens, cutaneous and general symptoms, current treatment, and histories of other allergic diseases. Patients who had abnormal vital signs or systemic symptoms were excluded from the study.
AD severity was evaluated by eczema area and severity index (EASI). Patients with scores of <16 were categorized as 'mild', those with scores between 16 and 26 were categorized as 'moderate' and those with scores of >26 were categorized as 'severe.'
A blood sample was collected from each subject. Serum total IgE levels were measured using the 3gAllergy IgE Test (Siemens, München, Germany) and serum food-specific IgE levels were measured using RIDA Allergyscreen (R-Biopharm AG, Darmstadt, Germany) or ImmunoCAP (Phadia, Uppsala, Sweden). Since RIDA Allergyscreen is a screening panel for dozens of foods, ImmunoCAP was used to measure serum food-specific IgE levels when the RIDA Allergyscreen showed a positive result.
Normal serum total IgE levels were different between age groups (1~4 years, 0.4~351.6 IU/ml; 5~10 years, 0.5~393.0 IU/ml; 11~14 years, 1.9~170.0 IU/ml; and >15 years, 0~378.0 IU/ml) according to the manufacturer's reference values. A positive response to a specific food was defined when serum food-specific IgE levels were >0.35 IU/ml according to the manufacturer's instructions.
We selected foods for open OFC testing based on food hypersensitivity history and serum food-specific IgE levels. Subjects who were negative for both history and serum food-specific IgE levels did not undergo open OFC testing. The skin lesions were prepared under optimal control, and medications that could interfere with the results were discontinued for 7~14 days10. Foods for challenge were prepared by parents or by physicians. Initial challenge doses were determined by physicians based on the kind of food, the patient's history and expected reactions10. The challenge doses were administered in gradual increments until they reached amounts in the regular diet. When subjects were suspected to have IgE-mediated food hypersensitivity, the interval of challenge testing was 15 minutes, and when they were suspected to have non-IgE-mediated hypersensitivity, it was 45 minutes10. When patients showed positive reactions to open OFC tests, such as urticaria, angioedema or exacerbation of existing eczema lesions, we stopped the challenge and checked for other cutaneous lesions and systemic reactions, including gastrointestinal or respiratory signs. In cases of no reaction, challenge testing was repeated the next day. The results were divided into early and late, or delayed reactions. The early reactions were defined as reactions occurring within 2 hours, while late or delayed reactions were defined as those occurring between 2 hours and 48 hours of the last challenge.
The results obtained from patients with and without food hypersensitivity as assessed by past histories and serum food-specific IgE levels were compared using a 2-by-k table with linear-by-linear association or the t test. Significance levels for all analyses were set at p<0.05. All statistical analyses were conducted using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA).
Forty-two (44.2%) of the 95 AD patients had a history of food hypersensitivity (Fig. 1). The most common suspicious foods reported by the patients or the patients' caregivers were egg (n=13, 13.7%), followed by pork (n=9, 9.5%), milk (n=8, 8.4%), snacks (n=5, 5.3%), beef, chicken, wheat, shrimp (n=3, 3.2% for each), chocolate, ice cream, fish, peanut (n=2, 2.1% for each) and buckwheat, crab, hamburger, ramen (n=1, 1.1% for each). There were 17 patients (17.9%) with other allergic diseases, including allergic rhinitis (n=10, 10.5%), asthma (n=6, 6.3%) and urticaria (n=3, 3.2%).
The mean EASI score of all patients was 16.05±9.76. Based on the EASI scores, 43 (45.3%) of the 95 patients were mild cases, 36 (37.9%) were moderate cases, and 16 (16.8%) were severe cases. The mean EASI score was higher in AD patients with a history of food hypersensitivity than in those without (16.71±10.28 versus 15.52±9.39), but the difference was not statistically significant (p=0.559). The symptoms were also more severe in patients with a history of food hypersensitivity than in those without (21.4% versus 13.2%), but the difference was not statistically significant (p=0.370) (Table 1).
The mean serum total IgE level was 237.69±168.52 IU/ml in patients with history of food hypersensitivity and 221.83±170.46 IU/ml in those without, but the difference was not statistically significant (p=0.652). The proportion of patients with high serum total IgE levels was also higher in patients with a history of food hypersensitivity than in those without (30.9% versus 26.4%), but the difference was not statistically significant (p=0.631).
Of the 95 patients, 39 (41.1%) showed elevated serum food-specific IgE levels (Fig. 1). Egg-specific IgE was highest (n=17, 17.9%), followed by milk (n=12, 12.6%), peanut (n=10, 10.5%), wheat (n=8, 8.4%), pork (n=6, 6.3%) and beef (n=5, 5.3%). However, specific IgE to crab, lemon/lime/orange, rice, shrimp, peach (n=3, 3.2%), garlic, onion (n=2, 2.1%), barley and fish (n=1, 1.1%) were not significantly elevated. Elevated serum food specific IgE levels showing more than 95% predictive were noted in 2 patients (nos. 1 and 2), suggesting IgE-mediated food allergy (Table 2).
Nineteen (45.2%) of the 42 patients with a history of food hypersensitivity showed elevated serum food-specific IgE levels, while 20 of the 53 (37.7%) patients without a history showed elevated serum food-specific IgE levels; the difference was not statistically significant (p=0.466) (Fig. 1).
Fifty-one (53.8%) of the 95 patients underwent 78 sessions of open OFC testing based on their history of food hypersensitivity (n=37) or serum food-specific IgE levels (n=31). Seven (13.7%) of these 51 patients were positive for open OFC testing. The remaining 44 patients did not undergo open OFC, and 33 of these patients were negative for both past histories and serum food-specific IgE levels, while 11 refused to undergo open OFC (Table 3) (Fig. 1). The overall prevalence of food hypersensitivity was 8.3% (7/84). The most common offending food was egg (n=3), followed by milk (n=2), pork (n=1) and peanut (n=1) (Fig. 2). Five (71.4%) of the 7 patients with positive open OFC test results showed a history of food hypersensitivity and elevated serum food-specific IgE levels, but 2 patients (28.6%) with positive open OFC test results did not show elevated serum food-specific IgE levels. Four patients (nos. 3, 5, 6 and 9) showed immediate non-eczematous reactions, while 2 patients (nos. 7 and 8) showed delayed eczematous reactions. One patient (no. 4) had both kinds of reactions. Two patients with highly elevated serum food-specific IgE levels (nos. 1 and 2) did not show any cutaneous reactions to open OFC testing.
Patients with both a history of food hypersensitivity and elevated serum food-specific IgE levels showed higher positivity rates for OFC testing (17.6%) than those with a history of food hypersensitivity alone (10%) or increased serum food-specific IgE levels alone (14.3%), but the differences were not statistically significant (p=0.511 and p=0.808, respectively) (Fig. 1). Five (9.8%) of the 51 patients who underwent open OFC testing had gastrointestinal symptoms and signs, including abdominal pain (n=3), vomiting (n=1), and diarrhea (n=1). Two of the 7 patients who were positive for open OFC testing had gastrointestinal symptoms. No patients showed any respiratory symptoms or signs during the open OFC testing.
Of the 13 patients with a history of egg allergy, 8 (61.5%) showed elevated serum egg-specific IgE levels. Nine (11.0%) of the 82 patients without a history of food hypersensitivity showed elevated serum food-specific IgE levels. Fifteen patients underwent open OFC testing for eggs, and 3 (20.0%) of these patients showed positive reactions to eggs. Patients (nos. 3, 4, and 5) who had both of a history of food hypersensitivity and elevated serum food-specific IgE levels tended to show early or mixed, but not delayed reactions. Of the 9 patients reporting a history of food hypersensitivity to pork, only 1 (11.1%) showed elevated serum pork-specific IgE levels. Five (5.8%) of the remaining 86 patients without a history of food hypersensitivity to pork showed elevated serum food-specific IgE levels. Twelve patients underwent open OFC testing for pork, of whom only 1 (8.3%) showed a positive reaction to pork. One patient with a positive reaction to open OFC testing (no. 8) had a history of food hypersensitivity without elevated serum food-specific IgE levels.
Serum milk-specific IgE levels were elevated in 5 (62.5%) of the 8 patients with a history of food hypersensitivity to milk, while they were elevated in 7 (8.0%) of the 87 patients without a history of food hypersensitivity. Two (16.7%) of the 12 patients who underwent OFC testing for egg showed positive reactions to egg; one patient (no. 6) with elevated serum food-specific IgE levels alone showed early reactions, while the other patient (no. 7) with a history of food hypersensitivity alone showed late reactions.
Peanut allergy was reported by 2.1% of patients with a history of food hypersensitivity and 10.5% of patients with elevated serum food-specific IgE levels. One patient (no. 9) who had positive reactions to open OFC testing did not report a history of peanut-related allergic reactions.
In 1915, Schloss3 first reported AD patients who improved by avoiding of offending food allergens. Since then, many studies about the relationships between AD and food hypersensitivity have been conducted4. It has been shown that food hypersensitivity plays a crucial role in the pathogenesis of AD. The prevalence of AD has been reported to be between 33% and 75%11,12. This wide range of prevalence is mostly due to differences in the definition of the food allergy in AD or the selection of severe AD patients who had been referred to a large hospital5,13.
There are 2 different reactions to foods in AD13,14. First, there are non-eczematous reactions, such as urticaria, erythema, and pruritus. These reactions are immediate or early, with IgE-mediated responses usually occurring within 1 hour, and may be accompanied by non-cutaneous reactions, such as gastrointestinal or respiratory symptoms. These features are common characteristics of food allergy. Second, there are isolated eczematous reactions that result in eczematous flare-ups. These reactions are late or delayed, with non-IgE-mediated responses usually occurring 2 to 6 hours or even up to several days after exposure to offending food allergens. The reactions may be T-cell mediated, IgE-independent reactions to food allergens15. In addition, there is a combination of non-eczematous and eczematous reactions. Previous studies have demonstrated that AD worsens with the intake of food additives or biogenic amines, which are not classical food allergens16. It is believed that these reactions are also due to IgE-independent hypersensitivity14.
Since IgE-mediated reactions are common, but eczematous reactions are relatively rare, the definition of food hypersensitivity, whether including immediate reactions or not, may cause marked differences in the prevalence of food hypersensitivity5,13. Previous studies have shown that late reactions alone are only responsible for 12% to 26% of positive challenge testing results, which is similar to 28.7% in our study17,18. There are still different viewpoints among dermatologists and allergists; the former limit food-induced AD strictly to eczematous responses, while the latter include both non-eczematous and eczematous responses13. In our study, there were 7 patients (8.3%) with positive reactions to open OFC testing, and 4 (4.2%) of these patients had non-eczematous reactions, 2 (2.1%) had eczematous reactions, and 1 (1.1%) had mixed reactions. Patients with immediate, non-eczematous reactions (nos. 3, 5, 6 and 9) had high serum food-specific IgE levels after challenge with food allergens, suggesting that these reactions are IgE-mediated. Of the patients with delayed, eczematous reactions, 2 (no. 7 and 8) did not show high serum total or food-specific IgE levels, implying 'true food-induced AD'. Only one subject (no. 4) showed a combination of urticaria and eczematous reactions along with a high serum food-specific IgE level.
The overall prevalence of food hypersensitivity in pediatric patients with AD in our study was lower than previously reported in Europe and North America14. Some previous studies that have enrollment AD patients have reported low prevalence of food hypersensitivity as in our study19. In our study, the prevalence of AD accompanied by other allergic diseases or non-cutaneous reactions, such as gastrointestinal or respiratory symptoms, was lower than those reported by previous studies8. Gastrointestinal and respiratory symptoms are related to IgE-mediated food allergy, accounting for a large proportion of food hypersensitivity in AD patients20. In addition, we included mild AD which is not included in previous studies21. In addition, the frequency of specific food allergy varies among different countries22. Previous studies have reported that egg and milk are the most common food allergens23, which is consistent with our result. Peanut allergy is relatively rare in Asia, although it has frequently been reported in Europe and North America24. In our study, the number of patients with elevated serum peanut-specific IgE levels was 5-fold higher than the number of patients with a history of peanut allergy, and 1 patient with no history of food hypersensitivity showed a positive challenge reaction to peanut. A previous study conducted in Korea has also reported that peanut allergy is rare in past history, but relatively common in open OFC testing or food-specific IgE measurement8. This suggests that the potential risk for peanut allergy exists, although its prevalence is lower in Korea than in Europe and North America. Many patients in our study considered pork an aggravating factor of AD, and most did not show elevated serum food-specific IgE levels or any positive reactions to open OFC testing. A previous epidemiologic study conducted in Korea has reported that pork is the most common food allergen in Korea (up to 10%)7. Another study conducted in Korea has shown that the common food allergens in AD patients in Korea are egg, cow's milk, peanut, and wheat, which is similar to our result, but that sensitization to pork is relatively unusual25. These results imply that having a pork allergy is highly overestimated by patients in Korea.
The criteria for the diagnosis of food allergies have not yet been established. The criteria should include clinical history, skin prick tests, serum IgE measurement, and atopy patch testing18. Skin prick tests and serum IgE measurement are useful for the diagnosis of early, IgE-mediated reactions, while atopy patch testing helps diagnose delayed reactions18. We used only serum IgE levels, because they are consistent with skin prick tests and atopy patch testing and they are relatively convenient to measure18. Double-blinded, placebo-controlled oral food challenge (DBPCFC) testing is the gold standard for the diagnosis of food hypersensitivity14. Open OFC testing is an alternative to DBPCFC for confirming clinical signs, such as cutaneous manifestations or wheezing, and it is easy to perform in the clinical setting10. We chose open OFC testing because preparation of various causative food allergens for DBPCFC is expensive and difficult. False positive reactions of subjective symptoms without objective signs, such as pruritus, are drawbacks to open OFC testing. Skin prick tests or serum IgE measurement may help interpret such reactions. When open OFC testing results are not conclusive, DBPCFC should be considered10.
When AD patients are suspected of having food hypersensitivity, their parents often restrict food. As seen in our study, the number of patients with a history of food hypersensitivity is 5-fold higher than those with actual food hypersensitivity according to open OFC testing (44.2% versus 8.3%). It has generally been recognized that milk-free foods are overemphasized considering the actual prevalence of milk allergy26. Dietary restriction without confirming food hypersensitivity has few clinical benefits and potentially causes nutritional deficiency. In our study, since the actual prevalence of food hypersensitivity in AD patients was not so high, parents should be advised by medical professionals before starting food restriction.
In conclusion, the overall prevalence of food hypersensitivity in childhood AD patients in Korea was 8.3%, as assessed by open OFC testing. The number of patients with actual food hypersensitivity was lower than the number of patients with a history of food hypersensitivity. Common offending food allergens in Korean AD patients were egg and milk. Among the food allergens causing hypersensitivity, peanut was underestimated, while pork was overestimated by patients with childhood AD in Korea.
Figures and Tables
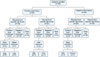 | Fig. 1Results of past history, serum food-specific IgE levels, oral food challenge testing, clinical reactions to oral food challenge, and causative food allergens. IgE: immunoglobulin E. |
 | Fig. 2Egg and milk were significant in positive past history, elevated serum food-specific immunoglobulin E (IgE) levels, and positive oral food challenge. Pork was significant in positive past history but not significant in elevated food-specific IgE levels and positive oral food challenge testing. Peanut was not significant in positive past history, although it was significant in elevated food-specific IgE levels and positive oral food challenge testing. |
ACKNOWLEDGMENT
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No.2011-0013003 and No.2011-0004436) and by a grant from Amore Pacific Co. Ltd., 2011 and by a grant from Hallym University Medical Center Research Fund (01-2012-01).
References
1. Kim JY, Lim HJ, Kim HY, Lee WK, Kim BS, Lee WJ, et al. Difference in the prevalence rate according to diagnostic criteria in atopic dermatitis: prevalence rate of atopic dermatitis according to Hanifin-Rajka, Japanese, Korean diagnostic criteria and characteristics of three different diagnostic criteria. Korean J Dermatol. 2010. 48:649–656.
2. Hahn EL, Bacharier LB. The atopic march: the pattern of allergic disease development in childhood. Immunol Allergy Clin North Am. 2005. 25:231–246.

3. Schloss OM. Allergy to common foods. Trans Am Pediatr Soc. 1915. 27:62–68.
4. Greenhawt M. The role of food allergy in atopic dermatitis. Allergy Asthma Proc. 2010. 31:392–397.

5. Rowlands D, Tofte SJ, Hanifin JM. Does food allergy cause atopic dermatitis? Food challenge testing to dissociate eczematous from immediate reactions. Dermatol Ther. 2006. 19:97–103.

6. Campbell DE. Role of food allergy in childhood atopic dermatitis. J Paediatr Child Health. 2012. 48:1058–1064.

7. Oh JW, Pyun BY, Choung JT, Ahn KM, Kim CH, Song SW, et al. Epidemiological change of atopic dermatitis and food allergy in school-aged children in Korea between 1995 and 2000. J Korean Med Sci. 2004. 19:716–723.

8. Jeon HJ, Bang HD, Kim KH, Park CW, Kim KJ, Lee CH. A Study of food allergy in atopic dermatitis using CAP-RAST FEIA and open food challenge test. Korean J Dermatol. 2003. 41:1034–1040.
9. Park YL, Kim HD, Kim KH, Kim MN, Kim JW, Ro YS, et al. Report from ADRG: a study on the diagnostic criteria of Korean atopic dermatitis. Korean J Dermatol. 2006. 44:659–663.
10. Nowak-Wegrzyn A, Assa'ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS. Adverse Reactions to Food Committee of American Academy of Allergy, Asthma & Immunology. Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009. 123:S365–S383.
11. Rancé F. Food allergy in children suffering from atopic eczema. Pediatr Allergy Immunol. 2008. 19:279–284.

12. Uenishi T, Sugiura H, Tanaka T, Uehara M. Role of foods in irregular aggravation of skin lesions in children with atopic dermatitis. J Dermatol. 2008. 35:407–412.

13. Suh KY. Food allergy and atopic dermatitis: separating fact from fiction. Semin Cutan Med Surg. 2010. 29:72–78.

14. Werfel T, Ballmer-Weber B, Eigenmann PA, Niggemann B, Rancé F, Turjanmaa K, et al. Eczematous reactions to food in atopic eczema: position paper of the EAACI and GA2LEN. Allergy. 2007. 62:723–728.

15. Abernathy-Carver KJ, Sampson HA, Picker LJ, Leung DY. Milk-induced eczema is associated with the expansion of T cells expressing cutaneous lymphocyte antigen. J Clin Invest. 1995. 95:913–918.

16. Worm M, Ehlers I, Sterry W, Zuberbier T. Clinical relevance of food additives in adult patients with atopic dermatitis. Clin Exp Allergy. 2000. 30:407–414.

17. Celik-Bilgili S, Mehl A, Verstege A, Staden U, Nocon M, Beyer K, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy. 2005. 35:268–273.

18. Roehr CC, Reibel S, Ziegert M, Sommerfeld C, Wahn U, Niggemann B. Atopy patch tests, together with determination of specific IgE levels, reduce the need for oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2001. 107:548–553.

19. Eigenmann PA, Calza AM. Diagnosis of IgE-mediated food allergy among Swiss children with atopic dermatitis. Pediatr Allergy Immunol. 2000. 11:95–100.

20. Roehr CC, Edenharter G, Reimann S, Ehlers I, Worm M, Zuberbier T, et al. Food allergy and non-allergic food hypersensitivity in children and adolescents. Clin Exp Allergy. 2004. 34:1534–1541.

21. Sicherer SH, Sampson HA. Food hypersensitivity and atopic dermatitis: pathophysiology, epidemiology, diagnosis, and management. J Allergy Clin Immunol. 1999. 104:S114–S122.

22. de Benedictis FM, Franceschini F, Hill D, Naspitz C, Simons FE, Wahn U, et al. EPAAC Study Group. The allergic sensitization in infants with atopic eczema from different countries. Allergy. 2009. 64:295–303.

24. Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007. 120:491–503.

25. Han YS, Chung SJ, Cho YY, Choi HM, Ahn KM, Lee SI. Analysis of the rate of sensitization to food allergen in children with atopic dermatitis. Korean J Community Nutr. 2004. 9:90–97.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download