Abstract
Background
Androgenetic alopecia (AGA) is characterized by the local and gradual transformation of terminal scalp hair into vellus hair, which has a shorter and thinner shaft. There are no studies that analyze annual changes in age, patterns, family history, and associated disease.
Objective
We investigated the severity of hair loss, age of onset, the frequency of family history, and past medical histories in Korean patients with AGA.
Methods
A retrospective chart review was performed to identify all patients with AGA referred to the Dermatology Clinic at Chung-Ang University Hospital from January 2006 to December 2010.
Results
The age of onset was also gradually decreased from 34.1±10.1 years to 31.6±10.9 years between 2006 and 2010. In female patients, specific annual changes were not observed. Hamilton-Norwood Type IIIv AGA was most common in male patients and Ludwig Type I AGA was most common in female patients at all times between 2006 and 2010. The majority of patients with AGA had a family history of baldness and was most commonly associated with a paternal pattern of inheritance. Seborrheic dermatitis was the most common associated disease in male and female patients.
Androgenetic alopecia (AGA) is the most common type of baldness, and is characterized by progressive hair loss. AGA is characterized by progressive thinning of the scalp hair. Both men and women can be affected by AGA. The density and diameter of hair in the involved area are both decreased1. Although the basic etiological factors in AGA are presumed to be the same in men and women, the phenotypic expression differs. Male pattern baldness is characterized by a receding hairline at the temples and balding of the vertex, which gradually enlarges to link together. In the female pattern, there is a diffuse decrease in density over the crown of the head and the frontal hairline is preserved2.
Androgen is a prerequisite for the development of AGA in genetically predisposed individuals3. It is generally believed that dihydrotestosterone is one of the pivotal mediators of hair loss in AGA. Baldness does not occur in men with an inherited 5-α-reductase deficiency4. Therefore, AGA is associated with increased scalp levels of dihydrotestosterone, which is derived from the conversion of testosterone by 5-α-reductase5,6.
There have been studies on the prevalence and patterns of AGA in Caucasians and Asians7. According to Hamilton's study8, by the age of 30 years approximately 30% of men will have AGA and this will increase to approximately 50% by 50 years of age. Female AGA affects up to 30% of older women9. However, there are no clinical studies of AGA that have analyzed annual changes in age, patterns of baldness, family history, and associated disease. Thus, we investigated the severity of hair loss, age of onset, frequency of familial history and past medical history in Korean patients with AGA referred to our outpatient clinic.
A retrospective chart review was performed to identify all patients with AGA referred to the Department of Dermatology Clinic at Chung-Ang University Hospital from January 2006 to December 2010.
Retrospective clinical examinations were performed and patient demographics, such as age, gender and family history, were obtained by reviewing medical records. To identify the age of onset of AGA, we subtracted the duration of the disease from the age of the patients at the time of attending our clinic. To assess the severity of hair loss, clinical photographs obtained at the first visit were analyzed and hair loss patterns were categorized using the Hamilton-Norwood classification for males and the Ludwig classification for females. The family history of AGA among first-degree relatives and previous generations was determined. The past medical history was also obtained by reviewing medical records.
The crosstabs and ANOVA test was used for statistical analysis of comparison between years.
From 2006 to 2010, a total of 1218 patients with AGA were referred to our outpatient clinic were analyzed data from 833 men and 385 women. Over the time period, the number of patients increased. The proportion of AGA patients among the new patients increased from 2006 to 2010. The proportion of AGA patients amongst all of the alopecia patients was similar between 2006 and 2010 (Table 1).
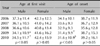
In male patients, the mean age at the time of the first visit gradually decreased from 37.3±11.4 years to 34.5±11.7 years between 2006 and 2010, respectively (p<0.05; Table 2). The age of onset also gradually decreased from 34.1±10.1 years to 31.6±10.9 years (p<0.05; Table 2). When analyzing the change of distribution of age at the time of the first visit, patients in 20s gradually increased in number and patients in 30s decreased year after year (p<0.05; Fig. 1A). However, no specific trends were observed with respect to the change of age at onset distribution (Fig. 1B). An analysis of age at the time of the first visit and the age of onset in females revealed no specific annual changes or trends in distribution (Table 2).
We analyzed AGA patterns in male and female patients using the Hamilton-Norwood and Ludwig classifications, respectively. In male patients, type IIIv AGA (31.2% [260 cases]) was most common between 2006 and 2010, the following: type II (17.8% [148 cases]), type III (13.5% [112 cases]), type IV (9.2% [77 cases]), type I (7.8% [65 cases]), type IIa (6.4% [53 cases]), type IIIa (5.6% [47 cases]), type V (2.4% [20 cases]), type IVa (2.3% [19 cases]), type Va (2.0% [17 cases]), female pattern (1.2% [10 cases]) and type VI (0.6% [5 cases]) (Table 3); 'female pattern' refers to a hair loss pattern that could not be categorized using the Hamilton-Norwood classification. These findings showed that the frontal hairline was preserved with only diffuse hair loss on the mid-frontal and parietal scalp, as shown by Ludwig's type I. Ludwig's type I AGA was most prevalent (75.8% [292 cases]) in females between 2006 and 2010, followed by type II (22.1% [85 cases]) and type III (2.1% [8 cases]; Table 4). In addition, we organized patterns by year between 2006 and 2010, then analyzed the annual changes in the AGA pattern. Type IIIv AGA was the most common type in male patients between 2006 and 2010 at all times. The percentage of patients with type II AGA increased year after year (p<0.05; Fig. 2A), but total of type I, type II and type IIa were not significantly increased (2006 [29.5%]; 2007 [33.5%]; 2008 [29.2%]; 2009 [32.0%]; 2010 [35.3%]). Type I AGA also increased gradually and type III AGA decreased, but the difference was not statistically significant. In the other types of AGA, specific changes were not observed. Type I AGA was most common in female patients between 2006 and 2010 at all times (Fig. 2B).
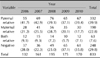
A family history of baldness was present in 70.2% (585/833) of male patients with AGA and in 66.2% (255/385) of female patients with AGA (Table 5, 6). Both male and female patients were most commonly associated with paternal inheritance. Specific annual changes were not observed (p<0.05).
Associated diseases were observed in 606 males (72.7%) and 276 females (71.7%; Table 7, 8). Seborrheic dermatitis was the most common associated disease in males (51.2% [310 cases]) and females (45.7% [126 cases]), followed in descending order by hypertension (male, 13.4% [81 cases]; female, 11.2% [31 cases]) and hyperlipidemia (male 5.9% [36 cases]; female 8.0% [22 cases]). Specific annual changes were not observed (p<0.05).
The term AGA was introduced to define hair loss under the influence of androgens against a background of genetically determined susceptibility with respect to the hair follicle2. AGA is considered pathological by some physicians, but as a normal variant of ageing by other physicians. The change in hair distribution on the scalp has been characterized in both genders. AGA in men produces patterned hair loss, beginning with bitemporal recession of the frontal hair line, followed by diffuse thinning over the vertex8. Over time, there is complete hair loss centrally on the vertex, producing a bald patch. Less commonly, men may develop 'female pattern' baldness, with preservation of their frontal hairline10. In contrast, women with AGA have hair loss more generally distributed over the top of the scalp, with preservation of the frontal hairline10. However, postmenopausal women, may develop male pattern baldness11.
Although AGA can affect all races, the prevalence and types of AGA vary between races and regions12. In Caucasians, the prevalence of AGA is 30% for men in the 4th decade, 40% for men in the 5th decade, and 50% for men in the 6th decade8. The results of studies conducted in the UK, USA, Italy, Norway and Australia support these findings with some variations1,13-16. Birch et al.1 reported that 6% of women <50 years of age and 38% >50 years of age had female pattern alopecia in the UK. Desmond et al reported that 44.9% of Australian men and 32.2% of women >20 years of age had AGA16. On the other hand, Khumalo et al.17 found that the prevalence of AGA was 14.6% in African men and 3.5% in African women, which is much lower than reported in Caucasians. In Koreans, the prevalence of AGA was 14.1% in men and 5.6% in women, which was much lower than in Caucasians and similar to the prevalence in Africans2. This was not a population-based study; the patients were referred to our dermatologic clinic. Therefore, we could not identify changes in the precise prevalence of AGA. However the number of AGA patients and the proportion of AGA patients among the new patients increased year after year. Although the proportion of AGA patients among all the patients with alopecia was similar between 2006 and 2010 (the average incidence of AGA among the alopecia patients over 5 years was 76.7%), The proportion increased more than previously reported by Han et al.18 (38.9%, from 1990 to 1993), Ro et al.19 (64.5% from 1995 to 1998) and Kim et al.20 (70.4% from 2001 to 2003) in Korea. This trend led us to speculate that the number of AGA patients to want treatment is increasing steadily. However, it is also possible that prevalence of AGA increases.
The prevalence of AGA increased steadily with advancing age. According to Paik et al.2, the prevalence of AGA was 2.3% in 3rd decade, 4.0% in 4th decade, 10.8% in 5th decade, 24.5% in 6th decade, 34.3% in 7th decade and 46.9% in the 8th decade and beyond in Korea. However, the distribution of patient seeking treatment of AGA was different, because older patients did not feel the need to treat AGA. In this study, patients in 3rd and 4th decades were predominant. This finding is similar to retrospective studies conducted by Han et al.18, Ro et al.19 and Kim et al.20 in Korea. The mean age of male patients at the time of the first visit decreased year after year in the current study. When analyzing the distribution of age at the time of the first visit, patients in the 3rd decade gradually increased and patients in the 4th decade decreased year after year. The average age of AGA patients increased and patients in the 3rd decade decreased in comparison to previous reports by Han et al.18 (mean age, 28 years; patients in the 2nd decade, 64.3% from 1990 to 1993) and Ro et al.19 (mean age, 27.5 years; patients in the 2nd decade, 65.7% from 1995 to 1998). Considering the increasing tendency in patients with AGA, however, the age of patients with AGA was not increased, but the proportion of older patients is thought to be increased because the older patients with AGA want to be treated by means of good economic environments. In this study, the mean age of onset AGA also significantly decreased in our study. However, most of studies analyzed age at first visit and didn't analyzed onset age. Therefore, we could not compare our results and previous studies. Our results show the possibility that the average age of onset is decreasing. The cause of this result is not clear. However, the possible elements that affect the changes in the age of onset of AGA include stressors due to the competitive structure of our society and increases in weight and body mass index due to the western style of eating habits. Acute or chronic stress as an aggravating factor in a hair loss disorder whose primary pathogenesis is of endocrine, toxic, metabolic or immunological nature21. Stress-induced hair growth inhibition is promoted by nervegrowth factor which appears to be up-stream of substance P21. González-González et al.22 reported that insulin resistance was significantly higher in AGA patients than controls. The insulin resistance was associated with eating habits23. The period of the present study was only 5 years, which is not sufficient for the precise determination of age of onset of AGA. However, this is the first study analyzing age of onset and can be the basis for another studies analyzing onset of AGA. Clearly, a long-term study is needed.
There are some useful classifications for male pattern baldness. Hamilton8 studied the developing patterns of scalp hair in men and women from the prenatal period through the 10th decade and divided balding patterns into 8 types with 3 subdivisions. Approximately 25 years later, Norwood revised Hamilton's classification by eliminating type III (a 'wastebasket' for uncertain diagnoses) and dividing the patterns into 7 with 4 subdivisions (I, II, III, IIIv, IIIa, IV, IVa, V, Va, VI and VII) in which the letter 'a' was applied to cases with advanced recession of the frontal hairline, and the term 'vertex' was applied to those with an isolated balding patch on the crown24. AGA in women was classified by Ludwig, who described 3 grades (I~III) with increasing severity of diffuse alopecia over the crown of the head, but without the frontoparietal recession that exists in men25. He defined grade I as a perceptible thinning of the hair on the crown with preservation of the frontal hairline, grade II as a pronounced thinning of the hair on the crown, and grade III as total baldness in the areas that exist in grades I and II. Recently, a new classification of AGA that is universal for men and women was introduced by Lee et al.26 in Korea. It is Basic and Specific (BASP) classification. BASP classification is a stepwise, systematic, and universal classification system for AGA. Because BASP was not yet widely used, we used Hamilton-Norwood classification for males and the Ludwig classification for females for comparing with another international study. A study of white Caucasian men revealed predominantly type II AGA8. A study involving Norwegian men 20~50 years of age reported type I as the commonest pattern (31%), followed by type II (26%) and type V or worse (20%)15. In studies involving Korean and Chinese men, type IIIv was the most common pattern2,7. As in the Korean and Chinese studies, type IIIv was the most common pattern in our study. When analyzing annual changes in the AGA pattern, types II increased gradually but total mild pattern (type I, type II and type IIa) did not increase significantly. Therefore, we could not conclude that mild pattern AGA increased in male patients. To identify annual changes in AGA pattern, a long-term study is needed.
AGA is considered a disease with a genetic predisposition, but the mode of inheritance has not been well-characterized. Osborn27 reported that AGA was inherited in an autosomal dominant manner in men; therefore, one would predict that sons should inherit the gene from the mother or the father in equal proportions. However, the strong concordance in baldness observed between fathers and sons is inconsistent with a simple Mendelian trait, and a polygenic mode of inheritance is considered most likely28. The heterogeneity in the clinical phenotype further suggests that AGA is inherited as a complex trait disorder. Ellis et al.29 reported that 81.5% of bald Caucasian sons had bald Caucasian fathers. In Korea, a family history of baldness was present in 48.5% of men and 45.2% of women. In Chinese, 29.7~55.8% of men and 19.2~32.4% of women had family histories of baldness2,7,12. In this study, 70.2% of men and 66.2% of women had a positive family history. Although specific annual changes of family history were not observed in our study, the family history were higher in each case than previously reported studies by Han et al.18 (male patients [39.4%], female patients [41.2%] from 1990 to 1993), Ro et al.19 (male patients [54%], female patients [52%] from 1995 to 1998) and Kim et al.20 (male patients [64.5%], female patients [59.4%], from 2001 to 2003) in Korea. An increased family history of baldness could be an indicator that reflects the increased prevalence of AGA, because AGA is considered a disease with a genetic predisposition. There have been a number of studies regarding associated diseases with AGA. In this study, associated diseases were observed in 72.7% of males and 71.7% of females. Seborrheic dermatitis was the most common disease in males (51.2%) and females (45.7%). The prevalence of seborrheic dermatitis is higher when compared to prevalence of general population (1~3%)30. the association between AGA and seborrheic dermatitis was not established definitely. However, it is known that seborrheic dermatitis is related to the elevated production of 5α-dihydrotestosterone (DHT) in affected areas31. Increased DHT plays an important role in seborrheic dermatitis, as well as AGA, because increased DHT activates the sebaceous gland32. Hypertension, hyperlipidemia, diabetes mellitus, and gastrointestinal diseases are common disease associated with AGA in descending order following seborrheic dermatitis. Recently, adult diseases have increased due to the adoption of Western style eating habits in Korea; we reasoned that this trend affected our results. Ahouansou et al.33 evaluated the association between androgenetic alopecia and hypertension; androgens which bind to mineralocorticoid receptors might be responsible for the high blood pressure and hyperaldosteronism which is considered to be responsible for most of primary hypertensions may directly participate in the development of alopecia. Men, who are between ages 19~50 years and who have early AGA (occurring before 35 years of age), have an increased incidence of hyperinsulinemia and disorders associated with insulin resistance, such as obesity, hypertension, and dyslipidemia34.
The limitations of our study were as follows: (1) the study was hospital-based rather community-based and was not a multicenter study; and (2) the study period was short for analyzing changes in age of onset, pattern, family history, and associated diseases of AGA. We plan to a long-term perform multicenter population-based study to identify changes in the age of onset, pattern, family, history and diseases associated with AGA.
Figures and Tables
 | Fig. 1Changes in distribution of (A) age at first visit and (B) onset age in male patients with androgenetic alopecia. |
 | Fig. 2Annual changes of clinical type in (A) male patients and (B) female patients with androgenetic alopecia. |
Table 2
Annual changes in mean age at first visit and onset age in male and female patients with androgenetic alopecia
References
1. Birch MP, Messenger JF, Messenger AG. Hair density, hair diameter and the prevalence of female pattern hair loss. Br J Dermatol. 2001. 144:297–304.

2. Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol. 2001. 145:95–99.

3. Tang L, Bernardo O, Bolduc C, Lui H, Madani S, Shapiro J. The expression of insulin-like growth factor 1 in follicular dermal papillae correlates with therapeutic efficacy of finasteride in androgenetic alopecia. J Am Acad Dermatol. 2003. 49:229–233.

4. Imperato-McGinley J, Gautier T. Inherited 5-α-reductase deficiency in men. Trends Genet. 1986. 2:130–133.
5. Kaufman KD. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol Clin. 1996. 14:697–711.

6. Drake L, Hordinsky M, Fiedler V, Swinehart J, Unger WP, Cotterill PC, et al. The effects of finasteride on scalp skin and serum androgen levels in men with androgenetic alopecia. J Am Acad Dermatol. 1999. 41:550–554.

7. Xu F, Sheng YY, Mu ZL, Lou W, Zhou J, Ren YT, et al. Prevalence and types of androgenetic alopecia in Shanghai, China: a community-based study. Br J Dermatol. 2009. 160:629–632.

8. Hamilton JB. Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. 1951. 53:708–728.

9. Norwood OT, Lehr B. Female androgenetic alopecia: a separate entity. Dermatol Surg. 2000. 26:679–682.

11. Venning VA, Dawber RP. Patterned androgenic alopecia in women. J Am Acad Dermatol. 1988. 18:1073–1077.

12. Wang TL, Zhou C, Shen YW, Wang XY, Ding XL, Tian S, et al. Prevalence of androgenetic alopecia in China: a community-based study in six cities. Br J Dermatol. 2010. 162:843–847.

13. Rhodes T, Girman CJ, Savin RC, Kaufman KD, Guo S, Lilly FR, et al. Prevalence of male pattern hair loss in 18-49 year old men. Dermatol Surg. 1998. 24:1330–1332.

14. Severi G, Sinclair R, Hopper JL, English DR, McCredie MR, Boyle P, et al. Androgenetic alopecia in men aged 40-69 years: prevalence and risk factors. Br J Dermatol. 2003. 149:1207–1213.

15. DeMuro-Mercon C, Rhodes T, Girman CJ, Vatten L. Male-pattern hair loss in Norwegian men: a community-based study. Dermatology. 2000. 200:219–222.

16. Gan DC, Sinclair RD. Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc. 2005. 10:184–189.

17. Khumalo NP, Jessop S, Gumedze F, Ehrlich R. Hairdressing and the prevalence of scalp disease in African adults. Br J Dermatol. 2007. 157:981–988.

18. Han ES, Kim MN, Hong CK, Ro BI. A clinical study of androgenetic alopecia. Korean J Dermatol. 1995. 33:44–52.
19. Ro BI, Ro SW, Shim JH. A clinical study of androgenetic alopecia (III). Ann Dermatol. 2002. 14:11–17.

20. Kim JE, Ahn JY, Ro BI. A clinical study of androgenetic alopecia (V). Korean J Dermatol. 2005. 43:319–324.
21. Hadshiew IM, Foitzik K, Arck PC, Paus R. Burden of hair loss: stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J Invest Dermatol. 2004. 123:455–457.

22. González-González JG, Mancillas-Adame LG, Fernández-Reyes M, Gómez-Flores M, Lavalle-González FJ, Ocampo-Candiani J, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol (Oxf). 2009. 71:494–499.

23. Gimeno SG, Andreoni S, Ferreira SR, Franco LJ, Cardoso MA. Assessing food dietary intakes in Japanese-Brazilians using factor analysis. Cad Saude Publica. 2010. 26:2157–2167.

24. Norwood OT. Male pattern baldness: classification and incidence. South Med J. 1975. 68:1359–1365.

25. Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977. 97:247–254.

26. Lee WS, Ro BI, Hong SP, Bak H, Sim WY, Kim do W, et al. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007. 57:37–46.

28. Chumlea WC, Rhodes T, Girman CJ, Johnson-Levonas A, Lilly FR, Wu R, et al. Family history and risk of hair loss. Dermatology. 2004. 209:33–39.

29. Ellis JA, Sinclair R, Harrap SB. Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med. 2002. 4:1–11.

30. Gupta AK, Bluhm R, Cooper EA, Summerbell RC, Batra R. Seborrheic dermatitis. Dermatol Clin. 2003. 21:401–412.

31. Kim BJ, Kim JY, Eun HC, Kwon OS, Kim MN, Ro BI. Androgenetic alopecia in adolescents: a report of 43 cases. J Dermatol. 2006. 33:696–699.

32. Yoo KH, Rho YK, Kim DH, Park J, Kim BJ, Kim MN, et al. A clinical study of androgenic alopecia (VII). Korean J Dermatol. 2009. 47:765–771.
33. Ahouansou S, Le Toumelin P, Crickx B, Descamps V. Association of androgenetic alopecia and hypertension. Eur J Dermatol. 2007. 17:220–222.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download