Abstract
Background
Methods
Results
Figures and Tables
 | Fig. 1Histopathological and immunohistochemical findings of biopsy specimens from normal control (a~e) and atopic dermatitis (AD) patients (f~j). H&E stained sections of the healthy skin of the normal control (a) and skin lesion of an AD patient (f). A variable degree of parakeratosis, acanthosis, spongiosis, and/or exocytosis, and sparse-to-moderate inflammatory cells infiltration were observed (×200). Immunohistochemical reactivity of staphylococcal protein A (SPA), recombinant staphylococcal enterotoxin A (SEA) in the skin of a healthy adult (b, c) and AD patients (g, h). Increased intensity of SPA, SEA immunoreactivity was observed in the upper part of the epidermis from all AD patients in comparison with that of a healthy adult (×200). Immunohistochemical reactivity of recombinant staphylococcal enterotoxin B (SEB) in the skin of a healthy adult (d) and AD patients (I). Minimal immunoreactivity of SEB was observed in the lesional skin of almost all AD patients (×200). Finally immunohistochemical reactivity of toxic shock syndrome toxin-1 (TSST-1) in the skin of a healthy adult (e) and AD patients (j). Mild to moderate immunoreactivity of TSST-1 was detected in the lesional skin of AD patients (TSST-1, ×200). |
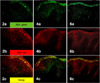 | Fig. 2Two-colored double-labeled immunofluorescent staining of SEA (a: green), SPA (b: red) and their merged image (c: yellow) in the epidermis of three atopic dermatitis patients (2, 4, 6). Co-localization of SEA and SPA was observed in merged images (yellow), suggesting the coexistence of SEA and Staphylococcus aureus itself in the epidermis of AD patients (2c, 4c, 6c) (2a~6c: ×200). SEA: staphylococcal enterotoxin A, SPA: Staphylococcal protein A, AD: atopic dermatitis. |
 | Fig. 3RT-PCR analysis for mRNA expression of the inflammation-related adhesion molecules in C, HaCaT cells (A, B) and HUVECs (C, D). The HaCaT cells and HUVECs were incubated for 6, 12, 24 and 48 hours after γSEA protein (100 ng/ml) treatment. (A, C) RT-PCR analysis for mRNA expression of E-selectin, ICAM-1, VCAM-1 and GAPDH was performed. (B, D) The density of each band was measured by a scanning densitometry and then expressed as the mean±standard deviation. The red box denotes significant upregulation of mRNA expression. M: marker, C: control, RT-PCR: reverse transcriptase-polymerase chain reaction, HUVECs: Human Umbilical Vein Endothelial Cells, γSEA: recombinant staphylococcal enterotoxin A. |
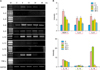 | Fig. 4RT-PCR analysis for mRNA expression of various cytokines after γSEA protein treatment in HaCaT cells. HaCaT cells were incubated for 6, 12, 24 and 48 hours after γSEA protein (100 ng/ml) treatment. (A) RT-PCR analysis for mRNA expression of various cytokines and GAPDH was performed. (B) The density of each band was measured by a scanning densitometry and then expressed as the mean±standard deviation. The red box denotes significant upregulation and the green box denotes minimal upregulation of mRNA expression. M: marker, C: control, IL: interleukin, TNF: tumor necrosis factor, RT-PCR: reverse transcriptase-polymerase chain reaction, γSEA: recombinant staphylococcal enterotoxin A. |
Table 1

Score: 0 (no clinical manifestation), 1 (mild), 2 (moderate), 3 (severe). AD: atopic dermatitis, E: erythema, I/P: induration/papulation, O: oozing, L: lichenification, SPA: Staphylococcal protein A, SEA: staphylococcal enterotoxin A, SEB: staphylococcal enterotoxin B, TSST-1: toxic shock syndrome toxin-1, NC: normal control, F: female, M: male, CD: chronic dermatitis, SAD: subacute dermatitis.
*Case number of 9 atopic dermatitis, †E-erythema, ‡(-): 0%, (+): <25%, (++): ≥25%.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download