This article has been corrected. See "Corrigendum: The Effects of 830 nm Light-Emitting Diode Therapy on Acute Herpes Zoster Ophthalmicus: A Pilot Study" in Volume 25 on page 400.
Abstract
Background
Skin lesions and pain are the most distinctive features of herpes zoster. Light-emitting diode (LED) therapy is an effective treatment known for its wound-healing effects.
Objective
To determine whether the LED treatment affects wound healing and acute pain in acute herpes zoster ophthalmicus.
Methods
We recruited 28 consecutive Korean patients with acute herpes zoster ophthalmicus for the study. In the control group (group A), 14 subjects received oral famcyclovir. In the experimental group (group B), 14 subjects received oral famcyclovir and 830 nm LED phototherapy on days 0, 4, 7, and 10. In order to estimate the time for wound healing, we measured the duration from the vesicle formation to when the lesion crust fell off. The visual analogue scale (VAS) was used for the estimation of pain on days 4, 7, 10, and 14.
Results
The mean time required for wound healing was 13.14±2.34 days in group B and 15.92±2.55 days in group A (p=0.006). From day 4, the mean VAS score showed a greater improvement in group B, compared with group A. A marginal but not statistically significant difference in the VAS scores was observed between the two groups (p=0.095).
Light-emitting diode (LED) therapy is known for its healing and anti-inflammatory properties. It is used in clinical practice as a supplement to other treatments, including nonablative thermal technology. Early animal and human studies have demonstrated the LED-induced diminution in wound size and protection from skin inflammation and ulceration1. Subsequent studies found that the positive effects of LED therapy were related to the stimulation of the fibroblast activity through the enhancement of the mitochondrial functions. In addition, the upregulation of procollagen synthesis in human fibroblast cultures and the downregulation of matrix metalloproteinases can be achieved by a variation of the LED fluencies and pulse duration2. To date, no studies have been published on the specific evaluation of the effects of LED for the treatment of herpes zoster. In this study, we used LED therapy for the treatment of acute herpes zoster ophthalmicus. Our aim was to determine whether the treatment of acute herpes zoster lesions with 830 nm LED therapy would result in a decrease of both the healing time and acute pain.
Following the approval by the local ethics committee (C2012020 [715]), 28 consecutive Korean patients with acute herpes zoster lesions localized to the V1 dermatome were randomized to the control or the experimental group (Table 1). The diagnosis of herpes zoster ophthalmicus was based on the typical eruption involving the dermatomal distribution of the ophthalmic division of the trigeminal nerve. We defined day 0 as the day that the vesicles began to appear. Patients were included only if they had no previous medical history known to influence wound healing, such as hypertension, diabetes mellitus, or immunosuppression. The control group (group A) included 8 men and 6 women, with a mean age of 54.50±4.85 years (range 47~62 years). The experimental group (group B) included 7 men and 7 women, with a mean age of 53.14±4.64 years (range 49~64 years). No statistically significant difference in age, gender or initial severity was observed between the two groups (p>0.05).
All patients in both groups received treatment with an antiviral agent (250 mg Famcyclovir) and analgesics three times a day for 7 days. Subjects in group A also received treatment with the conventional methods on days 0, 4, 7, and 10. The conventional treatment consisted of topical washing, cleaning of lesions, and removal of necrotic tissue, as appropriate. Subjects in group B received the conventional treatment and 830 nm LED phototherapy using HEALITE™ (Lutronic, Goyang, Korea) on days 0, 4, 7, and 10. Skin was exposed to 830 nm wavelength light at a setting of 55 mW/cm2 for 10 minutes twice per week.
For estimation of the healing time, the duration from the vesicle formation to when the lesion crust fell off was measured by the same independent blinded dermatologist. Pain was estimated on days 4, 7, 10, and 14. The 10-point visual analogue scale (VAS) (0 for no pain to 10 for very severe pain) was used for the measurement of pain.
For statistical analysis, an independent-samples t-test was used for a comparison of the mean healing time, and a repeated-measure of ANOVA test was used for a comparison of the difference in the VAS scores between the two groups. p≤0.05 were considered statistically significant. The SPSS 12.0 statistical package (SPSS Inc., Chicago, IL, USA) was used for the computation of all data.
The first aim of the study was to compare the healing time of the two treatment groups by the measurement of the duration from the vesicle formation to when the lesion crust fell off. The mean time needed for healing was 13.14±2.34 days in group B and 15.92±2.55 days in group A (p=0.006). Group B required significantly less healing time than group A (Fig. 1). The second aim of the study was to compare the level of acute pain using the VAS. From day 4, the mean VAS score showed greater improvement in group B, compared with group A. A marginal difference in the VAS scores was observed between the two groups (p=0.095) (Fig. 2).
Low-intensity light therapy, commonly referred to as photobiomodulation, using light in the far-red to near infrared region of the spectrum (630~1,000 nm), modulates numerous cellular functions. Low-power lasers and LEDs are the well-accepted therapeutic tools for use in the treatment of the infected, ischemic, and hypoxic wounds, along with other soft tissue injuries. At the cellular level, photobiomodulation can modulate fibroblast proliferation and attachment, as well as the synthesis of collagen. Using photobiomodulation, both the accelerated-healing and a greater amount of epithelization in the wound-closure of the skin grafts have been demonstrated in human studies. Findings in the literature also show that the LED therapy is known to support and speed up the healing of chronic leg ulcers, including the diabetic, venous, arterial, and pressure ulcers3,4.
We hypothesized that the positive effects of LED therapy on the wound healing would result in a more rapid healing and less scarring in herpes zoster lesions. A series of recent studies have demonstrated the anti-inflammatory effects of LED therapy. The findings from one study conducted with the arachidonic acid-treated human gingival fibroblast suggest that 635 nm irradiation inhibits prostaglandin 2 synthesis in a fashion similar to inhibition by cyclooxygenase inhibitors5. Another study found that LED therapy has beneficial effects on the prevention of post-inflammatory hyperpigmentation and scarring6. Irradiation at 830 nm has accelerated fibroblast transformation and mast cell degranulation. In addition, chemotaxis and phagocytic activity of leucocytes and macrophages is enhanced on cellular stimulation by this wavelength7. LED phototherapy with planar arrays of 830 nm diodes is delivered in a hands-free manner. It has been shown to be pain-free, side-effect-free, and well-tolerated by patients of all ages.
In our study, we were able to show that the patients treated with the LED phototherapy achieved faster healing and the lower mean VAS scores from day 4, compared with the control group. However, the difference in the VAS scores between the two groups showed no statistical significance. We think that the reason for the statistically-insignificant difference in the VAS scores was the small number of the enrolled patients. However, p=0.095 has a numerical value of marginal significance. Therefore, we believe that conducting a large scaled study may allow us to draw a statistically significant conclusion in the VAS score difference.
The pain reduction may have been the result of the anti-inflammatory effects and the improved wound-healing attributed to the LED therapy. Recent reviews concluded on the strong evidence of the low-level laser therapy modulating the inflammatory process and relieving acute pain in the short-term. Some of the potential mechanisms of action were the neurophysiologic effects, release of endogenous opioids, local microcirculatory and angiogenic effects, local anti-inflammatory effects, biochemical marker effects, and cell and soft tissue effects8,9. We believe that the use of this treatment will also result in a decreased risk of postherpetic neuralgia, which is correlated with acute pain. Although we were not able to determine a clear and precise mechanism of LED phototherapy, this therapy appears to be beneficial, not only for the treatment of chronic and non-healing wounds, but also in the management of acute herpes zoster lesions. In addition, the future double-blinded, large-scaled studies with a long-term follow up period is necessary in order to further evaluate the clinical benefits of LED in the treatment of acute herpes zoster.
Figures and Tables
Fig. 2
Repeated measures of mean VAS scores of the control and experimental groups from day 0 to 14. Differences in VAS were marginally significant between the two groups according to the repeated measure of ANOVA (p=0.095). VAS: visual analogue scale.
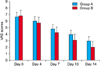
Fig. 3
The control group (group A). (A) Erythematous grouped vesicles appeared in a unilateral fashion following right ophthalmic division of the trigeminal nerve. (B) Three weeks after the LED treatment, the skin lesion moderately improved, but there still remained the erythematous scarring and hyperpigmentation. LED: light-emitting diode.

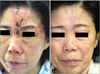
Fig. 4
The experimental group (group B). (A) Grouped vesicles and crust appeared in a unilateral fashion following left ophthalmic division of the trigeminal nerve, involving primarily the left forehead, which were extended to ipsilateral nose at the baseline. (B) 3 weeks after the LED treatment, the skin lesion markedly improved. LED: light-emitting diode.
ACKNOWLEDGMENT
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0008687).
References
1. Whelan HT, Smits RL Jr, Buchman EV, Whelan NT, Turner SG, Margolis DA, et al. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001. 19:305–314.

2. Vinck EM, Cagnie BJ, Cornelissen MJ, Declercq HA, Cambier DC. Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Lasers Med Sci. 2003. 18:95–99.

3. Minatel DG, Frade MA, França SC, Enwemeka CS. Phototherapy promotes healing of chronic diabetic leg ulcers that failed to respond to other therapies. Lasers Surg Med. 2009. 41:433–441.

4. Caetano KS, Frade MA, Minatel DG, Santana LA, Enwemeka CS. Phototherapy improves healing of chronic venous ulcers. Photomed Laser Surg. 2009. 27:111–118.

5. Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002. 3:214–220.

7. Russell BA, Kellett N, Reilly LR. A study to determine the efficacy of combination LED light therapy (633 nm and 830 nm) in facial skin rejuvenation. J Cosmet Laser Ther. 2005. 7:196–200.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download