Abstract
Dermal cells from neonatal mice can initiate the formation of hair follicles (HFs) when combined with adult mouse epidermal cells and transplanted subcutaneously into athymic mice. In the present study, the effects of dermal cells on HF formation were tested in terms of total cell number and the time course of cell harvest. Results demonstrated that the number of dermal cells is critical to the formation of HF. Furthermore, hair forming ability is rapidly decreasing as the neonatal mice age. To examine potential differences in gene expression, cDNA array was performed. Results demonstrate that numerous molecules which are directly involved in receptor and signaling correlated with decreased hair inductivity in early time points after delivery. It is reported that bone morphogenic protein (BMP)-6 and Wnt3a treatment increased hair inductivity of dermal papilla cells. But in our study, no changes were observed in the expression levels of BMP-6 and Wnt3a. However, several Wnt related genes demonstrate increased or decreased expression levels. Thus, our results suggest that co-ordinated regulation of these molecules will be important in hair neogenesis within our model system.
The hair follicle (HF) is composed of both epidermal and dermal compartments and the interaction between these cells has an important role in the morphogenesis and growth of the HF1. In general, dermal cells are considered the inducers and epithelial cells as responders in the process of hair formation2. For the regeneration of hair, several models have been established and recombination experiments have demonstrated that the HF unit can be induced through epithelial-mesenchymal interactions3. Using this model, epithelial-mesenchymal interactions were tested in order to determine the factors essential for the formation of HFs. It is known that cultured dermal papillar (DP) derived cells gradually lose their hair inductivity and proliferative capacity after being passaged in culture in vitro. Some success has been reported through sphere formation in prolonging the hair inductivity of cultured DP cells4. In addition to sphere formation, it has also been demonstrated that treatment with bone morphogenic protein (BMP)-6 or Wnt3a improved the formation of HF as compared to control DP cells5,6. In this study, DP cells gradually lost hair inductivity according to decreasing number of cells and postnatal age of the mice. Therefore, alterations in the expression levels of BMP and Wnt genes were compared between murine dermal cells from newborn mice and 7 days post-birth.
All animals were purchased from Japan SLC, Inc. (Shizuoka, Japan). Experimental protocols were approved by the Seoul National University Bundang Hospital (SNUBH) animal Institutional Review Board. C57BL/6 female mice (10±1 weeks old, Japan SLC, Inc.) were anesthetized with ketamine/xylazine. Full thickness skin was excised from the midback region of the mice. Excised skin was cut into 5×5 mm squares and incubated in 0.25% Trypsin (Gibco, Grand Island, NY, USA) at 4℃ for 16~18 hours. After removal of the dermal component, the epidermis was vortex-mixed and filtered through 70 µm mesh. Isolated cells were centrifuged at 800 rpm for 5 minutes. Dorsal skin was collected from newborn mice (C57BL/6) at different ages (postnatal day 0, 7, and 14). Harvested skin tissue was incubated in 0.25% Trypsin (Gibco) at 4℃ for 16~18 hours. After removal of the epidermis, the remaining dermis was cut into 5×5 mm pieces and incubated in 0.35% collagenase at 37℃ for 25 minutes. Following treatment, cells were filtered through a 100 µm mesh and centrifuged at 800 rpm for 5 minutes. Pellet (A) and supernatant (B) were further processed separately. First, pellet (A) was resuspended and re-centrifuged at 300 rpm for 3 minutes. The supernatant was collected and re-centrifuged at 800 rpm for 3 minutes. Pellet (A') was collected. Second, supernatant (B) was centrifuged at 1,400 rpm for 5 minutes. Following centrifugation, the pellet was resuspended and re-centrifuged at 800 rpm for 3 min. Pellet (B') was collected. Pellet (A') and pellet (B') were resuspended and mixed. After centrifugation at 800 rpm for 3 minutes, the pellet was recovered and filtered through a 30 µm mesh. Epidermal and dermal cell components to be grafted together were mixed in a sterile tube and pelleted by centrifugation. Cell pellets were kept on ice (1 to 2 hr) prior to grafting to the BALB/c nude mouse. Five-week-old nude mice were anesthetized and received different combinations of cells according to the number of cells or the age of donor mice. Total 4~8 million cells in 0.1 ml were injected by the subcutaneous bleb technique using 27 gauge needles. At the time of sacrifice, animals were photographed and the graft sites excised. Samples were fixed in 10% formalin and embedded in paraffin. Four µm sections were stained with hematoxylin and eosin and observed by light microscopy. The total number of HFs was calculated from each nodule developed at the injection site. Total RNA was extracted from dermal cells which were obtained from neonatal mice, day 7, and day 14 post-birth using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA, USA). The integrity of the total RNA and labeled cRNA were assessed by capillary electrophoresis on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Total RNA (550 ng) was amplified to cDNA, transcribed to cRNA and biotin labeled using the Illumina Total Prep RNA Amplification Kit (Ambion, Austin, TX, USA) following the manufacturer's instructions. Biotinylated cRNA (750 ng) was hybridized to Illumina Mouse Ref-8 v2.0 Expression BeadChips (Illumina, Inc., San Diego, CA, USA) according to the manufacturer's protocols. BeadChips were scanned with Illumina Bead Array Reader.
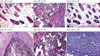
The mixture of neonatal mouse dermal cells (105~106 cells) and adult mouse epidermal cells (104~106 cells) was inoculated into the subcutaneous layer of BALB/c nude mouse. After 3 weeks, dark nodules appeared at the sites of inoculation (Fig. 1). Biopsy of the nodules and examination with light microscopy also revealed numerous HFs. Formation of HFs was dependent on the number of dermal cells and dramatically decreased with decreasing numbers of dermal cells from 106 to 105 (Fig. 2). HF formation was not observed when 104 cells were used. However, HF formation was induced with high numbers of dermal cells even though the number of epidermal cells was decreased from 106 to 104 (Fig. 2). These results showed that dermal cells are one of the main factors in the induction of HFs. Next, the effects of donor age on the ability to induce HF formation were tested. Dermal cells were collected from newborn mice, day 7, and day 14 day post birth. The mixture of epidermal cells (106) and dermal cells (106) was inoculated into the subcutaneous layer of the recipient mice. Three weeks post-inoculation, results demonstrate that HF formation is dramatically decreased depending on the age of the donor mouse (data not shown). cDNA analysis was performed to determine potential changes in gene expression between neonate and 7 day old mice using extracted total RNA (Table 1). Results indicated numerous genes exhibited more than a 2 fold difference in expression levels. However, no bone morphogenic protein family members including (BMP 1, 2, 3, 4, 5, 7, 8) demonstrated more than a 2-fold difference between neonate and 7 day old mice dermal cells. Wnt family members also demonstrated alterations in expression levels. The expression of Wnt 5a was found to be increased by 2.09 and Wnt7b decreased by 2.09. In addition, Wist1 was decreased.
HF stem cells in the epithelial bulge are responsible for the continued regeneration of the HF during cycling. The bulge cells reside in a niche composed of dermal cells. It has been established that the interaction between HF epithelial and dermal cells is necessary for HF morphogenesis in hair reconstitution assays2. Our results showed that inoculation of neonatal dermal cells and adult epidermal keratinocytes is sufficient to induce HF in mouse models. Because inoculation of adult dermal cells and adult keratinocytes cannot induce hair formation, our early findings revealed that neonatal dermal cells are key inducer of HF growth. The different combinations were inoculated to find their role in the induction of HF morphogenesis. Results demonstrate that the formation of HFs was dramatically decreased accordingly to the decreased number of dermal cells. However, the HF was well formed even with decreased numbers of epidermal cells if the optimized number of dermal cells is used simultaneously. These results suggest that dermal cells may produce factors which are critical in the formation of HFs. In our experiments, little to no hair formation was observed when less than 105 dermal cells were implanted. From the results, it can be concluded that threshold amounts of vital factors are necessary for the induction of the HF. Interesti ngly, the total number of epidermal keratinocytes was not critical if enough dermal cells were implanted simultaneously. These results showed that epidermal component is necessary but not critical in hair regeneration.
To examine the factors which are important in the induction of HF formation, the ability of dermal cells from donor mice of varying ages to induce hair were examined. In our study, HF forming ability of the dermal cells rapidly decreased with increasing age of the donor mice. cDNA arrays were performed to compare the gene expression profile between the newborn and day 7 post-birth mice. Results showed that the expression levels of a large number of genes are dramatically modulated within a few days post-birth. As expected, numerous process genes are altered which are correlated with the initiation of signal transduction pathways and developmental processes. Interestingly, 117 genes, which are associated with immunity and defense, are altered. These results imply that immunological factors are strongly involved in hair generation in early stages of murine development. In addition, numerous functional gene expression levels are changed, which encompass a variety of cell processed including receptors, signaling molecules, transcription factors, nucleic acid binding molecules, and extracellular matrix proteins. Further examination of the role of these factors is critical to determine the essential factors involved in hair generation.
In previously reported literature, it has been established that cultured DP cells gradually lose their hair inductivity and proliferative capacity after repeated passage in culture4. To increase the initiation of HF development, several methods have been utilized. First, DP cells cultured as spheres exhibit increased hair-inductivity when compared to cells cultured in 2-D cells. In addition, the DP spheres retain their hair inductivity with repeated passage in vitro4. It has also been reported that BMP-6-treated murine DP cells have improved HF formation capacity compared to control DP cells with retention of this phenotype up through passage 85. Therefore, changes in BMP expression were compared and examined. In our gene expression assay, expression of BMP-6 was not detected. In addition, other BMP family member expression levels were not significantly altered. Therefore, our results indicated that expression levels of BMPs are not dramatically modulated in early stages after delivery, even though BMP-6 treatment can increase the observed hair inductivity7. In addition, activation of Wnt signaling is known to be important for the initiation and maintenance of hair morphogenesis. In previously published reports, Wnt-3a treated DP cells demonstrate an increased capacity to induce HF formation as compared to untreated or sonic hedgehog (Shh)-treated DP cells6. Alternations in expression levels of Wnt family members were also examined. Similar to results obtained with BMP-6, the expression of Wnt3a is not altered significantly. However, the expression level of Wnt5a was increased and Wnt7b was decreased. It has been reported that addition of soluble Wnt3a and Wnt5a can induce BMP-4 and 6 expression8. Therefore, administration of Wnt3a may regulate the expression of BMP-6 which can be beneficial for hair growth6. Recently, it has been reported that Wnt5a is highly expressed by dermal papilla cells during the anagen phase9. It is likely that increased levels of Wnt5a expression in our model may be produced by anagen phase dermal papilla cells. In addition, our results suggest that Wnt5a is a dynamic factor within the hair cycle but further study will be necessary to determine the exact role of Wnt5a in the growth of hair. Finally, results indicate that the WNT1 inducible signaling pathway protein 1 (Wisp1) expression level was decreased. WISP1 is one of target genes of the canonical WNT signaling cascade10. All these results combined suggest that coordinated regulation of Wnt and BMP signaling will be important for the regeneration of hair.
In summary, our results demonstrate that the expression of dermal factors is rapidly changing during post-natal aging by the modulation of gene expression. Even though BMP-6 and Wnt-3a treatment increased hair inductivity of DP cells, our results suggest that coordinated regulation of these factors will be critical for hair neogenesis.
Figures and Tables
 | Fig. 1Formation of nodules at inoculation sites according to different combination of dermal (105~106 cells) and epidermal cells (104~106 cells). |
References
1. Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006. 126:1459–1468.

3. Chuong CM, Cotsarelis G, Stenn K. Defining hair follicles in the age of stem cell bioengineering. J Invest Dermatol. 2007. 127:2098–2100.

4. Osada A, Iwabuchi T, Kishimoto J, Hamazaki TS, Okochi H. Long-term culture of mouse vibrissal dermal papilla cells and de novo hair follicle induction. Tissue Eng. 2007. 13:975–982.

5. Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008. 22:543–557.

6. Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000. 14:1181–1185.

7. Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002. 118:216–225.

8. Dai J, Hall CL, Escara-Wilke J, Mizokami A, Keller JM, Keller ET. Prostate cancer induces bone metastasis through Wntinduced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res. 2008. 68:5785–5794.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download