Abstract
In a subgroup of patients suffering from atopic dermatitis (AD), treatment is quite difficult even after taking oral immunosuppressants. High-dose intravenous immunoglobulin (IVIG) treatment has been reported to be beneficial for them in a few uncontrolled trials. Herein we report a case of intractable AD in a 5-year-old girl who had significant clinical improvement after receiving 3 cycles of IVIG treatment (2 g/kg) without notable side effects. Since the first infusion of IVIG, the patient's skin lesions improved steadily and the improvement persisted until the 8-month follow-up. The eczema area and severity index score decreased remarkably, while immunologic parameters did not correlate with clinical improvement. This case suggests that IVIG therapy can be quite effective and safe for children with resistant AD.
Atopic dermatitis (AD) is one of the most common dermatologic diseases, affecting approximately 20% of children living in industrialized countries. Moreover, approximately 70% of cases of AD affect children less than 5 years of age1,2. Most cases of AD are effectively treated with topical steroids, topical calcineurin inhibitors and intermittent use of immunosuppressive agents, including oral corticosteroids and cyclosporine. However, for recalcitrant AD, continuous use of systemic immunosuppressive agents is used but is limited by the adverse effects, especially for children. For this subgroup of patients, a number of clinical trials based on immunomodulatory drugs have been conducted with limited success3-5, and high-dose intravenous immunoglobulin (IVIG) therapy has been reported to be effective in some recent trials6-12; in particular, children have shown much higher responses than adults6-8, and no serious side effects accompanied the therapy. In the current report, we present the case of a 5-year-old female with treatment-refractory AD who was remarkably improved with IVIG therapy without notable side effects. Her state of remission continued for 8 months without disease recurrence.
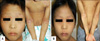
A 5-year-old female with a 3-year history of treatment-refractory AD was referred to our Pediatric Dermatology Clinic. She had failed to respond to 2 years of topical steroids and calcineurin inhibitors. Oral steroid treatments as an inpatient and outpatient during the last year were not much help. Recurrent staphylococcal infections and severe pruritus resulted in numerous hospital admissions. She was clinically depressed and needed a psychiatric consultation. She didn't show any symptoms of allergic rhinitis and asthma. There was also no family history of AD, allergic rhinitis and asthma. Quantitative measurement of the extent and severity of the AD was made using an eczema area and severity index (EASI) scoring system, the initial value of which was 30 (Fig. 1A). Laboratory results showed a total IgE of 11,000 kU/L and multiple allergen stimultaneous tests (RIDA Allergy Screening System, R-Biopharn, Darmstadt, Germany) were positive for D. farinae (grade 5), Alternaria (grade 4), house dust (grade 3), and cat (grade 3). The percentage of blood eosinophils was 15.2. She was first treated with cyclosporine (5 mg/kg) without significant benefits; the drug was discontinued after 3 months. After stopping cyclosporine treatment, the patient's state was slightly aggravated for 7 weeks. The EASI score was 32, which was its highest during the whole disease course. Then, IV-GLOBULIN (Greencross, Yongin, Korea) monotherapy was administered (2 g/kg monthly over 5 days) after hospital admission. Although she experienced mild nausea and headaches with every infusion, the skin symptoms subsided as the rate of infusion was decreased and oral antihistamines were administered. After 3 cycles of treatment with IVIG, the skin lesions improved dramatically (Fig. 1B) and the EASI score declined from 32 to 4 (Fig. 2). The total IgE level and blood eosinophil percentage were 9,000 kU/L and 15.3% each, which did not change significantly during clinical improvement. After 8 months of follow up in the outpatient clinic, the patient's AD remained in a state of remission; at that time she was taking a mild topical steroid and using an emollient.
IVIG has expanded beyond its traditional role as a treatment for primary immunodeficiencies, and is widely used in treating severe dermatologic diseases. IVIG has been increasingly used for dermatologic indications, including dermatomyositis, autoimmune bullous disorders, vasculitic syndromes, and toxic epidermal necrosis; the European Union guidelines have recently been suggested13. In addition to the above-mentioned indications, the high value of IVIG therapy in dermatologic diseases that are otherwise recalcitrant to conventional treatment is being consistently reported.
Regarding the clinical use of IVIG for AD, double-blind, placebo-controlled studies have not been conducted. One randomized study14 involving 9 adult patients with AD treated with a single cycle of IVIG monotherapy did not show significant clinical improvement. However, in several case reports and uncontrolled trials, the clinical efficacy of IVIG for children, have been consistently reported6-12. There have been 4 open trials of immunoglobulin treatment involving children with severe AD. Kimata6 administered IVIG (0.4 g/kg) to 4 AD patients, 2 of whom had underlying Kawasaki disease and the others had idiopathic thrombocytopenic purpura. All of the children improved in 4~7 days with sustained remission for 6 months. With clinical improvement, the serum IgE level and eosinophil count also decreased. Huang et al.7 reported that 5 children with severe AD and no other underlying diseases received 3 cycles of IVIG (2 g/kg), and all had marked improvement. The remission persisted over 6 months without relapse. Other immunologic markers, including ICAM-1, ELAM-1, ECP, and IL-2R decreased accordingly. Noh and Lee8 reported that patients with recalcitrant AD showed clinical improvement to variable degrees after receiving a single cycle of relatively low-dose IVIG (patients weighing <30 kg were treated with 500 mg/kg of IVIG and those weighing >30 kg were treated with 15 g of IVIG) over a 3-week follow-up period. The only case which did not show clinical improvement after receiving IVIG (1 g/kg) was an 8-month-old boy with Wiskott-Aldrich syndrome9.
In our case, the patient received 3 cycles of IVIG (2 g/kg) and had dramatic clinical improvement without recurrence for 8 months and without notable side effects. Our treatment regimen was similar to that in the study of Huang et al.7, while the immunologic parameters did not change significantly before and after IVIG treatments. Comparing with previous trials, our patient showed a more pronounced clinical response and maintained a longer prolonged remission state. Although the specific dosage regimens and follow-up periods were different in the above trials and our case, we can draw the following conclusions tentatively: 1) a few cycles of IVIG monotherapy is clinically effective in pediatric patients with refractory AD, with an early clinical response and prolonged remission; 2) no significant side effects were observed in the trials; 3) immunologic parameters reflected clinical improvement in some cases. While both reporting bias for successful outcomes and the small number of total patients in clinical trials should be taken into account carefully, IVIG monotherapy is considered especially useful for children with severe AD. This could be explained by the fact that the immunoregulatory function of IVIG is more profound in the relatively immature immune system of children, although further research is required for confirmation.
Compared with other immunosuppressants for recalcitrant AD, the safety profile of IVIG appears to be outstanding. Most of the reported side effects are mild and self-limiting15 including headache, low grade fever, nausea, vomiting and flushing. Side effects of IVIG vary from individual-to-individual and often occur 30~60 min after the onset of the infusion. These side effects are usually overcome by reducing the infusion rate and administering antihistamines or non-steroidal anti-inflammatory drugs before beginning the infusion. In our case, the patient had headaches, nausea, and fevers immediately after the start of all three infusions, which resolved after slowing the infusion rate and taking oral antihistamines. In very rare cases, severe side effects, such as aseptic meningitis, stroke, and anaphylactic shock, might occur during the infusion, especially for patients with underlying risk factors. Therefore, performing a medical work-up is important and adverse effects can be minimized by following the guidelines in the physician's checklist16. About possible mechanisms of IVIG treatment, modulation of T helper 1 (Th1) and Th2 cytokine production is suggested as one of the possible mechanisms. Intracellular IL-4 decreases after infusing high-dose IVIG17. However, considering the general conversion of cytokine profiles from Th2 to Th118 in late-stage AD, other mechanisms, such as recirculation of skin homing T cells and blockade of provoking antigens should also be considered.
In conclusion, we herein report a case in which IVIG monotherapy was very helpful in treating resistant AD in a child, without significant side effects. We also found that the remission period after treatments was longer than that of previous reports.
Figures and Tables
References
1. Williams HC. Clinical practice. Atopic dermatitis. N Engl J Med. 2005. 352:2314–2324.
2. Williams HC, Wüthrich B. Williams HC, editor. The natural history of atopic dermatitis. Atopic dermatitis: the epidemiology, causes, and prevention of atopic eczema. 2000. Cambridge, United Kingdom: Cambridge University Press;41–59.

3. Grundmann-Kollmann M, Podda M, Ochsendorf F, Boehncke WH, Kaufmann R, Zollner TM. Mycophenolate mofetil is effective in the treatment of atopic dermatitis. Arch Dermatol. 2001. 137:870–873.
4. Jang IG, Yang JK, Lee HJ, Yi JY, Kim HO, Kim CW, et al. Clinical improvement and immunohistochemical findings in severe atopic dermatitis treated with interferon gamma. J Am Acad Dermatol. 2000. 42:1033–1040.

5. Jacobi A, Antoni C, Manger B, Schuler G, Hertl M. Infliximab in the treatment of moderate to severe atopic dermatitis. J Am Acad Dermatol. 2005. 52:522–526.

6. Kimata H. High dose gammaglobulin treatment for atopic dermatitis. Arch Dis Child. 1994. 70:335–336.

7. Huang JL, Lee WY, Chen LC, Kuo ML, Hsieh KH. Changes of serum levels of interleukin-2, intercellular adhesion molecule-1, endothelial leukocyte adhesion molecule-1 and Th1 and Th2 cell in severe atopic dermatitis after intravenous immunoglobulin therapy. Ann Allergy Asthma Immunol. 2000. 84:345–352.

8. Noh G, Lee KY. Intravenous immune globulin (i.v.IG) therapy in steroid-resistant atopic dermatitis. J Korean Med Sci. 1999. 14:63–68.

9. Weiss SJ, Schuval SJ, Bonagura VR. Eczema and thrombocytopenia in an 8-month-old infant boy. Ann Allergy Asthma Immunol. 1997. 78:179–182.

10. Jolles S, Hughes J, Rustin M. The treatment of atopic dermatitis with adjunctive high-dose intravenous immunoglobulin: a report of three patients and review of the literature. Br J Dermatol. 2000. 142:551–554.

11. Wakim M, Alazard M, Yajima A, Speights D, Saxon A, Stiehm ER. High dose intravenous immunoglobulin in atopic dermatitis and hyper-IgE syndrome. Ann Allergy Asthma Immunol. 1998. 81:153–158.

12. Jolles S, Sewell C, Webster D, Ryan A, Heelan B, Waite A, et al. Adjunctive high-dose intravenous immunoglobulin treatment for resistant atopic dermatitis: efficacy and effects on intracellular cytokine levels and CD4 counts. Acta Derm Venereol. 2003. 83:433–437.

13. Enk A. European Dermatology Forum Guideline Subcommittee. Guidelines on the use of high-dose intravenous immunoglobulin in dermatology. Eur J Dermatol. 2009. 19:90–98.

14. Paul C, Lahfa M, Bachelez H, Chevret S, Dubertret L. A randomized controlled evaluator-blinded trial of intravenous immunoglobulin in adults with severe atopic dermatitis. Br J Dermatol. 2002. 147:518–522.

15. Gelfand EW. Differences between IGIV products: impact on clinical outcome. Int Immunopharmacol. 2006. 6:592–599.

16. Jolles S, Hughes J, Whittaker S. Dermatological uses of high-dose intravenous immunoglobulin. Arch Dermatol. 1998. 134:80–86.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download