Abstract
Onychomycosis is usually caused by dermatophytes, but some species of nondermatophytic molds and yeasts are also associated with nail invasion. Aspergillus niger is a nondermatophytic mold which exists as an opportunistic filamentous fungus in all environments. Here, we report a case of onychomycosis caused by A. niger in a 66-year-old female. The patient presented with a black discoloration and a milky white base and onycholysis on the proximal portion of the right thumb nail. Direct microscopic examination of scrapings after potassium hydroxide (KOH) preparation revealed dichotomous septate hyphae. Repeated cultures on Sabouraud's dextrose agar (SDA) without cycloheximide produced the same black velvety colonies. No colony growth occurred on SDA with cycloheximide slants. Biseriate phialides covering the entire vesicle with radiate conidial heads were observed on the slide culture. The DNA sequence of the internal transcribed spacer region of the clinical sample was a 100% match to that of A. niger strain ATCC 16888 (GenBank accession number AY373852). A. niger was confirmed by KOH mount, colony identification, light microscopic morphology, and DNA sequence analysis. The patient was treated orally with 250 mg terbinafine daily and topical amorolfine 5% nail lacquer for 3 months. As a result, the patient was completely cured clinically and mycologically.
Onychomycosis is caused mainly by dermatophytes but occasionally by nondermatophytic fungi including Scopulariopsis brevicaulis, Aspergillus spp., Fusarium spp., and Acremonium spp.1-3. In the past, these molds have been regarded as saprophytic or opportunistic fungi and have been basically ignored. Recently, as a consequence of an increase in the number of cases of immunodepression and environmental changes, more attention has been given to this wide, but generally non-pathogenic group of fungi4.
Onychomycosis caused by nondermatophytic molds is becoming increasingly prevalent even in healthy people. This apparent emergence might be an artifact of improved diagnostic techniques or increased awareness that these fungi are potential etiologic agents5. Onychomycosis cases caused by Aspergillus spp. are reportedly 2.6% to 6.1% worldwide6-8. With regard to onychomycosis caused by Aspergillus niger, several studies have been conducted4,9-12, and 2 cases have been reported by Tosti and Piraccini13.
Here the authors present the case of a 66-year-old woman diagnosed with onychomycosis caused by A. niger based on clinical findings, potassium hydroxide (KOH) test, fungal culture, light microscopy findings, and molecular analysis.
A 66-year-old female presented with black discoloration on the right first, third, and fourth fingernails and left second and third fingernails (Fig. 1). Patient history revealed that the nail lesions had first developed 2 months prior. The subject was otherwise in good health and denied nail trauma or dystrophic nail abnormalities prior to the onset of the present lesions. Upon clinical examination, the right first, third, and fourth fingernails and left second and third fingernails had a black discoloration with a milky white base. In the case of the right first fingernail, periungual inflammation was observed around the proximal portion, as well as onycholysis. There were no lesions on her hands, feet, or toenails and past medical history and family history were unremarkable. Physical examination, determined her general physical condition to be well with no specific findings other than a skin lesion. Laboratory studies, including a complete blood cell count with differential, peripheral blood smear, liver, renal function, and venereal disease research laboratory tests, urinalysis, stool examination, viral hepatitis and human immunodeficiency virus tests, chest X-ray, and electrocardiogram, were all within normal limits or negative. Direct microscopic examination of nail specimens showed the presence of dichotomous septate hyphae (Fig. 2). Cultures were subsequently grown in 2 Sabouraud's dextrose agar (SDA) slants without cycloheximide that were incubated at 25℃ for one week. A large number of the same colonies were observed to grow rapidly in the 2 slants. Initially the growths appeared whitish but turned black with time. The reverse surface of the slants turned yellowish. There was no colony growth on SDA slants with cycloheximide. Likewise, the same findings were observed in a subculture using SDA plates (Fig. 3, 4). Repeated cultures of nail plate samples taken 3 times at 2 week intervals all yielded similar findings. Upon microscopic examination with lactophenol cotton blue staining, hyaline septate hyphae and biseriate phialides covering the entire vesicle with radiate conidial heads were observed (Fig. 5). For molecular biologic analysis, DNA was extracted from the cultured colonies and the base sequence of the internal transcribed spacer (ITS) was identified. Subsequently, it was compared to the base sequence of A. niger strain ATCC 16888 (GenBank accession number AY373852) which was stored in Genbank, using the Basic Local Alignment Search Tool (BLAST) program. The result was a 100% match (Fig. 6). Using clinical and mycological results as well as molecular biological findings, we confirmed the diagnosis of A. niger. The patient was treated orally with 250 mg terbinafine daily and topical amorolfine 5% nail lacquer for 3 months.
Onychomycosis usually caused by dermatophytes and nondermatophytic mold and yeast, accounts for approximately 50% of onychopathic1 diseases. Onychomycosis caused by nondermatophytic molds comprises 1.45% to 17.6% of total cases, of which Aspergillus, Scopulariopsis, Fusarium, and Acremonium may be the cause4,8,11,14,15.
There are approximately 900 species in the genus Aspergillus which are common in the soil and decaying vegetation throughout the world, but are also found in all types of organic debris. Among these 900 species, A. fumigatus, A. flavus, A. niger, A. terreus, A. glaucus, A. chevalieri, A. ustus, and A. nidulans are known to cause infections in the human body16.
Aspergillus spp. are not keratinophilic, unlike dermatophytes, and thus cause onychomycosis through secondary infections after an external injury or previous disease17. However, several studies have reported Aspergillus spp. as a primary cause of onychomycosis13,18. The incidence rate of onychomycosis caused by Aspergillus spp. has been described as 2.6% to 6.1%, varying depending upon the reporter6-8. In Korea, the incidence rate of onychomycosis caused by Aspergillus spp. has been reported to be 4.6%, based on an account by Lim et al.10. This variation may reflect geographic differences in mold distribution, differences in the criteria used for diagnosing mold onychomycosis, and use of mycologic methods inappropriate for mold growth11. A. niger, an opportunistic filamentous fungus, mostly causes otomycosis or aspergillomas, but has been reported to cause skin infections, endophthalmitis, endocarditis, and onychomycosis as well19,20.
Since Aspergillus spp. are common laboratory contaminants, it is difficult to discern between the pathogen and the contaminant when Aspergillus spp. are identified in onychomycosis. According to English21, nondermatophytic mold can be considered as a pathogen of onychomycosis only when hyphae or spores are seen on microscopic examination and the same strain is identified through repeated cultures.
Recently, DNA sequencing of the ITS region has proved useful in corroborating the diagnosis in difficult cases of onychomycosis17,22.
In the case of A. niger, a nondermatophytic mold, dichotomous septate hyphae are observed on KOH test13,17,23. Additionally, colonies rapidly grow in culture and initially appear whitish but turn black with time. When these colonies are stained with lactophenol cotton blue, hyaline septate hyphae and biseriate phialides covering the entire vesicle with radiate conidial heads are microscopically observed15,23.
In our case, dichotomous septate hyphae were observed on KOH test, and the same findings were observed in repeated culture tests. Moreover, the same findings were observed with lactophenol cotton blue stain. The base sequence of ITS was identified from DNA extracted from the cultured colonies and subsequently compared to the base sequence of A. niger strain ATCC 16888 (GenBank accession number AY373852) stored in GenBank, using the BLAST program. The result was a 100% match.
With regard to onychomycosis caused by A. niger, several studies have been conducted4,9-12, and 2 cases have been reported by Tosti and Piraccini13. A comparison made between our case and the 2 cases of Tosti and Piraccini13 (Table 1) showed that all 3 cases occurred in adults over age 40 and lasted 2 to 6 months. Tosti and Piraccini's cases13 involved men's toenails, while our case occurred in a woman's fingernails. The patient had grown bean sprouts for an extended period of time and thus her hands were regularly exposed to water. As such, her job is presumed to be closely related to the disease pathogenesis. All 3 patients had no tinea pedis or tinea manus and also had no history of trauma.
In 1998 Baran et al.24 classified onychomycosis into 5 clinical types: 1) distal lateral subungual onychomycosis (DLSO), 2) superficial white onychomycosis, 3) proximal subungual onychomycosis (PSO), 4) endonyx onychomycosis, and 5) total dystrophic onychomycosis. DLSO is the most common type, and PSO is known to be comparatively rare19. Nevertheless, Tosti and Piraccini's cases13 were PSO with painful inflammation of periungual tissues. Furthermore, our case was also PSO with periungual inflammation.
In Tosti and Piraccini's cases13, black pigment existed in the toenails and black pigment was observed in the fingernail lesions in our case. This discoloration was caused by the black conidia of A. niger in nail keratin, which clinically suggests a diagnosis of onychomycosis. In all 3 cases, dichotomous septate hyphae were observed and colonies of A. niger grew only in SDA without cycloheximide.
It is known that onychomycosis caused by nondermatophytic mold does not respond well to common treatment25. However, Tosti et al.11 applied topical and systemic treatment to cases of onychomycosis caused by Aspergillus spp., and 5 out of 7 patients were completely cured. Thus, they found that onychomycosis caused by Aspergillus spp. responded better to treatment than other nondermatophytic molds. Gianni and Romano8 administered oral terbinafine to 34 patients with onychomycosis caused by Aspergillus spp. at 500 mg a day, using 1 week administration alternated with a 3 week suspension over 3 months. As a result, the cure rate reached 88%. In Tosti and Piraccini's 2 cases13, oral terbinafine was administered 250 mg a day for 3 months leading to a complete cure. Likewise, in our case, oral terbinafine was administered 250 mg/day in addition to the local application of amorolfine 5% nail lacquer. As a result, the patient was completely cured clinically and mycologically.
In conclusion, we should consider onychomycosis caused by A. niger in cases where black pigment around the proximal portion of nails with periungual inflammations is observed without tinea pedis or tinea manus, and when dichotomous septate hyphae are observed on KOH test. Apparently such symptoms respond well to antifungal medication and should be treated appropriately.
Figures and Tables
 | Fig. 1Black discoloration with milky white base and onycholysis on the proximal portion of the right thumb nail. |
 | Fig. 2Direct microscopic examination of scraping from a potassium hydroxide (KOH) preparation revealing dichotomous septate hyphae (KOH mount, ×400). |
 | Fig. 3Multiple, black colonies with velvety surfaces on Sabouraud's dextrose agar slants after 1 week at 25℃. |
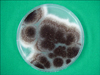 | Fig. 4A rapidly growing, black colony with a velvety surface on an Sabouraud's dextrose agar plate after 5 days at 25℃. |
 | Fig. 5Biseriate phialides covering the entire vesicle with radiate conidial heads in a slide culture of Aspergillus niger (lactophenol-cotton blue stain, ×400). |
References
1. Verma S, Heffeman MP. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Superficial fungal infection: dermatophytosis, onychomycosis, tinea nigra, piedra. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1807–1821.
3. Bassiri-Jahromi S, Khaksar AA. Nondermatophytic moulds as a causative agent of onychomycosis in tehran. Indian J Dermatol. 2010. 55:140–143.

4. Gianni C, Cerri A, Crosti C. Non-dermatophytic onychomycosis. An understimated entity? A study of 51 cases. Mycoses. 2000. 43:29–33.

5. Gupta AK, Ryder JE, Baran R, Summerbell RC. Non-dermatophyte onychomycosis. Dermatol Clin. 2003. 21:257–268.

6. Romano C, Gianni C, Difonzo EM. Retrospective study of onychomycosis in Italy: 1985-2000. Mycoses. 2005. 48:42–44.

7. Gupta M, Sharma NL, Kanga AK, Mahajan VK, Tegta GR. Onychomycosis: clinico-mycologic study of 130 patients from Himachal Pradesh, India. Indian J Dermatol Venereol Leprol. 2007. 73:389–392.

8. Gianni C, Romano C. Clinical and histological aspects of toenail onychomycosis caused by Aspergillus spp.: 34 cases treated with weekly intermittent terbinafine. Dermatology. 2004. 209:104–110.

9. Hilmioğlu-Polat S, Metin DY, Inci R, Dereli T, Kilinç I, Tümbay E. Non-dermatophytic molds as agents of onychomycosis in Izmir, Turkey-a prospective study. Mycopathologia. 2005. 160:125–128.

10. Lim SW, Suh MK, Ha GY. Clinical features and identification of etiologic agents in onychomycosis (1999-2002). Korean J Dermatol. 2004. 42:53–60.
11. Tosti A, Piraccini BM, Lorenzi S. Onychomycosis caused by nondermatophytic molds: clinical features and response to treatment of 59 cases. J Am Acad Dermatol. 2000. 42:217–224.

12. Surjushe A, Kamath R, Oberai C, Saple D, Thakre M, Dharmshale S, et al. A clinical and mycological study of onychomycosis in HIV infection. Indian J Dermatol Venereol Leprol. 2007. 73:397–401.

13. Tosti A, Piraccini BM. Proximal subungual onychomycosis due to Aspergillus niger: report of two cases. Br J Dermatol. 1998. 139:156–157.

14. Summerbell RC, Kane J, Krajden S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses. 1989. 32:609–619.

15. Rippon JW. Medical mycology: the pathogenic fungi and the pathogenic actinomycetes. 1988. 3rd ed. Philadelphia: WB Saunders;618–650.
16. Böhler K, Metze D, Poitschek C, Jurecka W. Cutaneous aspergillosis. Clin Exp Dermatol. 1990. 15:446–450.

17. Takahata Y, Hiruma M, Sugita T, Muto M. A case of onychomycosis due to Aspergillus sydowii diagnosed using DNA sequence analysis. Mycoses. 2008. 51:170–173.

18. Grover S. Clinico-mycological evaluation of onychomycosis at Bangalore and Jorhat. Indian J Dermatol Venereol Leprol. 2003. 69:284–286.
19. De Hoog GS, Guarro J, Gene J, Figueras MJ. Atlas of clinical fungi. 2000. 2nd ed. Utrecht: Centraalbureau voor Schimmelcultures;442–519.
20. Suh MK, Ha GY. A clinical and mycological study of otomycosis. Korean J Med Mycol. 1999. 4:15–20.
22. Makimura K, Tamura Y, Mochizuki T, Hasegawa A, Tajiri Y, Hanazawa R, et al. Phylogenetic classification and species identification of dermatophyte strains basedon DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Clin Microbiol. 1999. 37:920–924.

23. Kwon-Chung KJ, Bennet JE. Medical mycology. 1992. Philadelphia: Lea & Febiger;201–247.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download