Abstract
Background
Bullous pemphigoid (BP) is an autoimmune subepidermal bullous disease associated with autoantibodies against BP180 and BP230. Enzyme-linked immunosorbent assay (ELISA) is a sensitive tool for the detection of immunoglobulin G (IgG) anti-BP180 and anti-BP230 autoantibodies.
Objective
The aim of this study was to evaluate the usefulness of ELISA for diagnosing and monitoring the disease activity of BP.
Methods
We evaluated serum IgG levels of anti-BP180 and anti-BP230 autoantibodies in 47 BP patients, 16 epidermolysis bullosa aquisita patients, and 15 healthy volunteers using ELISA. Through retrospective review of the medical records, the clinical characteristics of BP including disease activity, duration, pruritus severity and peripheral blood eosinophil counts were assessed.
Results
The sensitivity of BP180 ELISA was 97.9%, BP230 ELISA 72.3%, and a combination of the two was 100%. The specificity of BP180 ELISA was 90.3%, BP230 ELISA 100%, and a combination of the two was 90.3%. BP180 ELISA scores showed strong associations with disease activity, pruritus severity, peripheral blood eosinophil counts, and disease duration, whereas BP230 ELISA scores did not.
Bullous pemphigoid (BP) is an autoimmune subepidermal bullous disease that is clinically characterized by generalized tense blisters arising on apparently normal or erythematous skin and even mucous membranes1. Immunopathologically, BP has been characterized by tissue-bound and circulating immunoglobulin G (IgG) autoantibodies against the antigen on the basement membrane zone (BMZ) of stratified epithelia2. Direct immunofluorescence (IF) study of perilesional skin reveals linear deposition of IgG and/or C3 along the BMZ. Indirect IF study using salt split skin demonstrates serum IgG autoantibodies bound to the epidermal side of the salt split skin. These autoantibodies in BP are known to react with two components of the hemidesmosome, BP230 and BP1803,4.
BP180, also known as type XVII collagen or BP antigen 2, is a 180 kDa transmembranous glycoprotein that ultrastructurally spans the lamina lucida before kinking back from the lamina densa into the lamina lucida. It comprises a globular cytoplasmic domain and a long C-terminal extracellular component consisting of 15 discontinuous collagenous domains separated by 16 non-collagenous (NC) domains5-7 (Fig. 1A). This protein contributes to the assembly and stabilization of hemidesmosomes8,9. The 16th NC (NC16A domain of the BP180 ectodomain, the NC16A domain, has been identified as the immunodominant region of BP180 in patients with BP, mucous membrane pemphigoid (MMP), and pemphigoid gestationis (PG)10-14.
The other major target antigen in BP is BP230 (also called BPAG1), a 230 kDa intracellular constituent of the hemidesmosomal plaque. BP230 belongs to the plakin family proteins and is considered to play an important role in the structural stability of hemidesmosomes. It comprises a central α-helical coiled-coil region flanked by two globular end domains and several distinct subregions15,16 (Fig. 1B). The N-terminal and C-terminal domains of BP230 are considered to be important in interactions with other hemidesmosomal transmembrane proteins such as BP180, β4 chain of α6β4 integrin, and keratin intermediate filaments, whereas the central α-helical coiled-coil domain apparently plays a role in its structural stability and self-assembly17-19. In addition to BP, reactivity to BP230 may be found in patients with paraneoplastic pemphigus and less frequently in those with MMP and PG12,20-22.
Autoimmune blistering dermatoses comprise various diseases that are characterized by autoantibodies to structural components of the skin and mucous membranes. For immunobullous disorders, accurate diagnosis is important because each of them shows different prognosis and requires specific treatment modality. Nevertheless, in most cases, an autoimmune bullous disease cannot be diagnosed based on clinical and/or histologic finding alone and requires the detection of tissue-bound and circulating autoantibodies. Indirect IF study using normal human skin or monkey esophagus substrates is the currently accepted method for detecting autoantibodies in BP. However, the method greatly depends on the operator's skill and is difficult to standardize. Immunoblotting and immunoprecipitation can also be used to identify the target antigens. However, they are both time-consuming qualitative techniques and thus are not suitable for routine screening of a large number of serum samples.
Enzyme-linked immunosorbent assay (ELISA) was performed on 96-well plates to allow 48 samples to be tested in duplicate. This assay is fairly simple, requiring only 5 µM of serum and is easily completed within a day, allowing the analysis of a large number of samples in a relatively short time. The data are objective as the optical density (OD) of each well is automatically read and expressed as a numerical value from a continuous scale. Similar to immunoblotting and immunoprecipitation, but unlike indirect IF, ELISA data provides information on antigen identity and allow differentiation between autoimmune subepidermal blistering diseases. In addition, it has the advantage of providing reproducible, quantitative data. Quantitative data from this assay may ultimately prove to be beneficial in guiding patient management.
Since synthetic or recombinant BP180 and BP230 fragments have become available, ELISAs based on recombinant proteins such as the NC16A domain alone or associated with other epitopes have been developed for the detection of these autoantibodies in patient sera23-27. The majority of BP sera have been revealed to react with the BP180-NC16A domain, and ELISA systems using recombinant BP180 had sensitivity ranging from 80% to 95%28. Furthermore, the levels of circulating IgG autoantibodies in BP patients detected via ELISA have been linked to the clinical activity of the disease25,29-31. BP230 ELISA has also been developed, and its sensitivity in BP patients ranges from 60% to 70%32-34. An extensive serological study utilizing an ELISA system with baculovirus-derived eukaryotic BP230 recombinant proteins confirmed that both the N-terminus and C-terminus of BP230 are major immunodominant regions targeted by the IgG autoantibody in BP35.
The purpose of this study was to evaluate the two commercially available ELISA systems (MBL Co., Nagoya, Japan) for detection of autoantibodies of BP180 and BP230 practically as routine diagnostic tests for BP and to address the potential relationship between autoantibody profiles and distinct clinical features and severity in BP patients.
We retrospectively evaluated serum samples from 47 patients (20 men, 27 women; mean age 71.5±14.7, range 30~94) who were diagnosed with BP and treated between May 2008 and November 2010 at the Department of Dermatology, Yonsei University College of Medicine. At the time of sample collection, 17 patients were on systemic immunosuppressive treatment. BP was diagnosed on the basis of the following criteria: characteristic clinical findings of pruritic plaques and tense blisters of the trunk and extremities without formation of scars or milia; histological evidence of subepidermal blisterings with numerous infiltrating polymorphonuclear cells, mainly eosinophils, along the basement membrane and within the blister cavities; evidence of linear deposits of IgG and/or C3 at the dermal-epidermal junction by direct IF; and evidence of circulating anti-basement membrane autoantibodies by indirect IF, more specifically, evidence of their deposit on the epidermal side of salt split skin. All patients' sera showed circulating IgG bound to the epidermal side of the salt split skin with titers ≥1:10. Forty-two BP patients presented skin lesions of BP without any mucous membrane involvement, and five other BP patients showed both skin and mucosal lesions without residual scarring. Sera from 15 healthy volunteers (9 males, 6 females) and 16 epidermolysis bullosa aquisita (EBA) patients (5 males, 11 females), whose diagnosis was confirmed by clinical criteria, routine histology, immunopathology, and ELISA reactivity with type VII collagen, were tested.
Serum samples were obtained from 5 successfully treated BP patients before treatment and after complete remission. "Complete remission" was defined as the absence of skin lesions for more than 1 month. Serial serum samples at 3 different time points during systemic treatment were collected from one patient, and were evaluated for autoantibody titers against BP180 and BP230 by ELISA to investigate the relevance between antibody titers and clinical disease activity.
To investigate the relationship between BP180 and BP230 ELISA scores and the disease characteristics of BP, serological, pathological, and clinical records of BP patients were examined. Among 47 BP patients, two patients were excluded for insufficient information on their medical records. With regards to disease characteristics, we examined four different variables: disease activity, disease duration, pruritus severity, and peripheral blood eosinophil counts.
To assess clinical disease activity of BP, we used a scoring system which was a modified version of a pemphigus scoring system designed by Herbst and Bystryn36. Disease activity was calculated by adding scores for the extent of disease and intensity of treatment at the time of sample collection. The disease extent was graded from 0 to 6+ based on the number of body areas involved. With the involvement of the oral mucosa, a score of 1+ was assigned. The intensity of therapy was graded from 0 to 5+ based on the corticosteroid dose and combination therapy required to control the disease. A score from 0 to 3+ was given according to the dose of corticosteroids (expressed in mg/day of methylprednisolone). If a combination therapy such as minocycline, nicotinamide powder or dapsone was used, a score of 1+ was assigned. If treatment with pulsed methylprednisolone or intravenous immunoglobulin was done, a score of 2+ was recorded. The disease activity of BP patients was graded on a 1~13 scale. BP disease severity was further classified as mild, moderate or severe based on the disease activity score of 1~4, 5~8 or 9~13, respectively (Table 1). The mean disease activity score of 45 BP patients was 6.8. The mild disease group included 10 patients, moderate disease group included 23 patients, and the severe disease group included 12 patients. The interval between the onset of symptoms and the time when the serum samples were obtained was defined as the disease duration. In 45 BP patients, the mean disease duration was 316.7±699.1 (range, 7~4,380) days. To compare the ELISA scores, BP patients were initially divided into two groups based on disease duration (<3 months and ≥3 months) as attempted in a previous report25. Owing to the lack of satisfactory methods for quantifying pruritus severity, the severity of pruritus was scored based on the dose of antihistamine drug, which was prescribed to control pruritus at the time of sample collection, as follows: none (0) when no antihistamine drug was prescribed, mild (1) when one or two kinds of antihistamine drug were prescribed, and severe (2) when more than two kinds of antihistamine drug were prescribed. The no pruritus group included 19 patients, mild pruritus included 19 patients, and severe pruritus group included 7 patients.
Peripheral blood eosinophil count was also evaluated at the time of sample collection. Peripheral blood eosinophil count was elevated in 17 BP patients (38%). The mean blood eosinophil count of 45 BP patients was 1020.6±1557.5 (range, 0~7330)/µl.
Anti-BP180 and anti-BP230 IgG autoantibody titers were measured using commercially available ELISA kits (MBL Co.) which are coated with recombinant proteins encompassing either the C- and N-terminus domain of BP230 or the NC16A domain of BP180. Following manufacturer's instruction, serum samples were diluted to 1:100 in assay diluent. Diluted serum samples were added to the wells. After rinsing off any unbound substances, peroxidase-conjugated goat polyclonal anti-human IgG antibodies were added to the wells. Development was performed using a microplate reader set to 450 nm to reflect the level of anti-BP180 and anti-BP230 autoantibodies bound in the initial step in each sample.
To compare results from different plates, test sample ODs were adjusted relative to the positive and negative control samples supplied in each kit. The mean OD of duplicate wells was calculated. The index value of each tested serum was defined by the following formula: index=(OD of tested serum-OD of negative control)/(OD of positive control-OD of negative control)×100. A cutoff ELISA value of 9 U/ml was used (≥9.0 U/ml being a positive result) on the basis of the manufacturer's recommendation.
The diagnostic accuracy of the ELISA in terms of the sensitivity and specificity was presented. ELISA scores were plotted by disease duration, disease severity grade and pruritus severity grade groups and compared between groups using Wilcoxon rank-sum test. The relationships of ELISA scores with other clinical variables were measured by Spearman correlation coefficient (r). Group comparison for categorical variables was done by the chi-square test or Fisher's exact test. All statistical analyses were performed by SAS version 9.2 (SPSS Inc., Chicago, IL, USA) using the alpha level of 0.05 to denote significance.
To assess the reactivity of BP sera and control sera against the BP180 and BP230, 47 BP sera, 16 EBA sera and 15 sera from normal healthy individuals were tested using commercially available ELISA kits. No association was observed between age or sex and between ELISA scores in BP patients and controls. Fig. 2 shows BP180 and BP230 ELISA scores in BP and control sera. BP180 ELISA was positive in 46 (97.9%) patients with BP with a cut-off value of 9.0, which was previously determined by the manufacturer. Thirty-four (72.3%) BP patients were positive for BP230 ELISA. Of all BP patients, 13 (27.7%) were positive for only BP180 ELISA, one (2.1%) was positive for only BP230 ELISA and 33 (70.2%) were positive for both BP180 and BP230 ELISA. No BP patient was negative for both BP180 and BP230 ELISA. In the control group, one healthy volunteer and two EBA patients were positive for BP180 ELISA, and all control sera were negative for BP230 ELISA. The false positive sera of the control group showed low concentrations of ELISA score (18, 13.9 and 9 U/ml) compared to the positive sera of BP patients. The sensitivity and specificity of BP180 ELISA were 97.9% (46/47) and 90.3% (28/31), respectively. Those for BP230 were 72.3% (34/47) and 100% (31/31). When the results of BP180 ELISA and BP230 ELISA were combined, sensitivity and specificity reached 100% (47/47) and 90.3% (28/31), respectively. These results are summarized in Table 2.
To determine clinical relevance, BP180 and BP230 ELISA scores were evaluated with respect to clinical characteris. BP180 ELISA scores exhibited a significant correlation with disease activity scores (r=0.45, p=0.002, Fig. 3A) and peripheral blood eosinophil count (r=0.34, p=0.02, Fig. 3C), while BP230 ELISA scores did not (Fig. 3B and D). In addition, BP180 ELISA scores were significantly higher in BP patients with shorter disease duration (<3 months) compared to those with longer disease duration (≥3 months) (p=0.02, Fig. 4A). Between these two groups of patients, disease activity scores and presence of treatment showed no significant difference (data not shown). There was also a significant relationship between BP180 ELISA scores and the grade of disease severity (p=0.006, Fig. 4C) and pruritus severity (p=0.04, Fig. 4E). In contrast, BP230 ELISA scores did not show any significant relationship with the clinical characteristics of BP (Fig. 4B, D and E). We compared disease duration, disease severity grade, and pruritus severity grade between BP patients who were positive for both BP180 and BP230 and patients who were positive for only BP180, using the chi-square or Fisher's exact test (Table 3). Between the two groups, there was no significant difference in disease duration, disease severity grade, and pruritus severity grade. One BP patient was positive only for the BP230 ELISA, and this patient did not show any different clinical features compared to other BP patients.
To evaluate the usefulness of ELISA and indirect IF as indicators for BP disease activity, we compared the indirect IF titers and ELISA scores before treatment and after complete remission in five BP patients. After remission, three patients (patients 1~3) showed marked reductions in indirect IF titer and in both BP180 and BP230 ELISA scores. Patient 4 showed marked reductions in BP230 ELISA score and indirect IF titer, but BP180 ELISA score was constantly negative throughout the study. Patient 5 showed marked reductions in BP180 and BP230 ELISA scores, but indirect IF titer was constant (Fig. 5).
We also compared ELISA scores, indirect IF titers, and disease activity along the time course with patients whose serial sera and clinical records were available. The treatment began with methylprednisolone (>30 mg/day) in addition to combination therapy such as mycophenolate mofetil (2.0 g/day), minocycline (100 mg/day), and dapsone (100 mg/day). After 2 years, ELISA scores and indirect IF titers decreased along with disease activity scores. In the following 2 months, although the ELISA scores and disease activity were nearly constant, the indirect IF titer increased from 1:10 to 1:40. In our patient, while BP180 and BP230 ELISA scores fluctuated along with disease activity, indirect IF titers did not (Fig. 6).
The pathogenic relevance of autoantibodies against BP180 has been supported by several findings in both in vivo and in vitro studies. First, passive transfer of rabbit IgG antibodies, raised against an extracellular region of murine BP180 homologous with the human immunodominant NC16A domain into neonatal mice induces all key features of BP37. Second, in PG, a disease closely related to BP occurring during pregnancy, the transplacentar transfer of anti-BP180 autoantibodies from the mother into the neonate can cause a transient bullous eruption38. Third, the titer of autoantibodies against BP180 appears to be related to the activity and extent of the disease24,25,29-31. Based on these findings, it is considered that autoantibodies to the extracellular domain of BP180 are pathogenically critical in the development of BP. Several clinical and experimental studies suggested that anti-BP230 antibodies may also play an important role in the onset of clinical symptoms and the formation of blisters39. First, rabbits immunized with BP230-derived peptides developed antibodies to the antigen, and, upon exposure to UVB light, they exhibited an enhanced inflammatory response, with IgG and C3 deposition at the BMZ40. Second, autoantibodies against BP230 were readily detected in the majority of patients, including those with relatively short disease duration. Third, neonatal mice that received subcutaneous injection high-titer BP230-specific polyclonal rabbit IgG exhibited inflammatory response and skin fragility41. These findings suggest that autoantibodies against BP230 may bind to the target antigen in injured keratinocytes or even in intact ones by penetrating the cells, and possibly contribute to subepidermal blister formation and perpetuation of the disease41. However, a role of autoantibodies against intracellular domains of BP230 and BP180 in the development of BP has not been fully elucidated.
In this study, we examined the sensitivity and specificity of the BP180 and BP230 ELISA systems using BP sera along with two different control sera-EBA and normal sera. Since the antigen proteins in these ELISA systems are not in full length, there was a possibility that autoantibodies against other domains could not be detected. Nevertheless, similar to previous reports23, the BP180 ELISA showed positive reactivity in 46 (97.9%) of 47 BP sera samples. In contrast, only one serum sample out of 16 EBA sera and two out of 15 normal control sera were positive, indicating the specificity of BP180 ELISA to be 90.3%. The frequency of reactivity against BP180 in our BP sera was higher than previously reported ELISA sensitivities ranging from 80% to 94%23-25,27. This discrepancy may be attributed to the number of BP patients tested, the percentage of treated versus untreated patients, and the disease extent related to anti-NC16A IgG levels25,29-31. We also examined the reactivity of the BP230 ELISA in BP sera, EBA sera and normal control sera. The BP230 ELISA showed positive reactivity in 34 (72.3%) out of the 47 BP sera samples. In contrast, all EBA and normal control sera were negative, indicating 100% specificity. Most importantly, when the results of the BP230 ELISA and BP180 ELISA were combined, the sensitivity rose to 100.0% (n=47). According to our data, all BP patients could be diagnosed using both the BP180 and BP230 ELISA.
BP180 ELISA scores showed a significant relationship with disease activity, pruritus severity, peripheral blood eosinophil count, and disease duration, whereas BP230 ELISA scores did not. Previous studies demonstrated a correlation between BP180 ELISA and disease activity of BP29,42,43. Peripheral blood eosinophil count was also significantly correlated with BP180 and BP230 ELISA scores44. In contrast, statistical analysis of our study demonstrated that BP180 ELISA scores relate to the blood eosinophil count, whereas BP230 ELISA scores do not. Such discordant findings require further study. The relationship between pruritus severity and titers of BP180 and BP230, however, has never been demonstrated before. High peripheral blood eosinophil counts and severe pruritus are considered characteristic features of BP. Therefore, our findings provide further evidence of BP180 as an important pathogenic factor in BP. In addition, we also showed the relationship between the BP180 ELISA score and disease duration. Patients with clinical lesions of BP persisting for 3 months or longer demonstrated significantly lower BP180 ELISA scores compared to those who presented clinical lesions of BP for less than 3 months. The two groups showed no significant difference in disease activity and presence of treatment. Based on this finding, we suggest that autoantibody against the NC16A domain of BP180, located at the extracellular portion of BP180, may initiate and worsen the disease. After initiation of the disease, the exposure of the cytoplasmic proteins from damaged basal keratinocytes is suggested to boost production of autoantibodies against the cytoplasmic epitope of BP180 and BP230 based on the finding of an increase in anti-BP230 autoantibody levels in proportion to disease duration and during disease progression44. In contrast, since BP230 ELISA scores did not show a significant correlation with disease duration in our study, we concluded that the production of anti-BP230 has no relevance to disease progression.
We compared ELISA scores and indirect IF titers before treatment and after complete remission in five BP patients. Three of them (patients 1~3) showed a significant decline in both ELISA scores and indirect IF titers at complete remission. Patient 4 was negative for BP180 ELISA before treatment and after complete remission. Interestingly, instead of the BP180 ELISA, the BP230 ELISA score decreased in parallel with the indirect IF titer. A similar BP patient with autoantibodies against BP230 but not for BP180 has been reported in a previous study44. Based on the finding that the BP230 score decreased along with disease activity score in this patient, IgG anti-BP230 antibody may play a pathogenic role in these exceptional BP cases. Further study with a large number of BP patients with positivity to only BP230 is necessary to elucidate the pathogenic role of BP230. Patient 5 showed a significant decline in the ELISA score, but the indirect IF titer was constant. We also evaluated three serial sera taken from one BP patient at different time points, and compared them with clinical disease activity. The BP180 and BP230 ELISA scores tended to fluctuate in parallel with the disease activity score along the time course and reflected disease severity, whereas the indirect IF titer did not. These observations suggest that when compared to indirect IF, which is subjective and known to depend greatly on the operator's skill, ELISA could be a sensitive diagnostic tool and a sensitive monitoring tool for disease activity. Furthermore, recurrence was more likely to occur in patients who had high BP180 ELISA scores earlier24,29,31,43. Based on these findings, BP180 ELISA scores may be helpful to plan tapering schedules of corticosteroids and to predict flares or relapses by detecting increases in antibodies before clinical evidence of disease flares are noticed. In addition to BP180 ELISA scores, BP230 ELISA scores also tended to fluctuate along with disease activity scores along the course of the disease in our study. Further study is needed to confirm our finding of the role of BP230 ELISA in monitoring disease activity of BP.
Our data show that currently available commercial ELISA kits for detection of autoantibodies against BP180 and BP230 have optimal diagnostic accuracy. Both methods have very high specificity, and BP180 ELISA, in particular, is more sensitive than BP230 ELISA. However, several BP patients showed negative findings for BP180 or BP230 ELISA. This finding may be explained by the existence of additional epitopes in BP180 and BP230. Therefore, to improve diagnostic sensitivity of ELISA, additional epitopes of the intracellular and extracellular portions of BP180 and BP230 should be included in the antigen molecules of ELISA. Based on thorough statistical analysis, our findings demonstrate that BP180 ELISA scores relate to the disease activity of BP, whereas BP230 ELISA scores do not. In addition to disease activity, BP180 ELISA score showed significant correlation with pruritus severity and peripheral blood eosinophil count, which are characteristic clinical features of BP. We also found that the ELISA scores showed parallel fluctuation with the disease activity along the time course, whereas the indirect IF titer did not.
Our findings may enhance the role of anti-BP180 autoantibody in the pathogenesis of BP, particularly by revealing the positive relationship between BP180 ELISA scores and several typical features of BP, including increased peripheral blood eosinophil count and pruritus severity. Although BP230 ELISA showed lower sensitivity than BP180 ELISA, BP230 ELISA was helpful in improving the sensitivity when used in combination with BP180 ELISA.
In conclusion, the ELISA systems for the detection of reactivity to both BP180 and BP230 are useful diagnostic and perhaps even monitoring tools for BP and may replace indirect IF using skin sections.
Figures and Tables
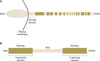 | Fig. 1Schematic diagrams depicting the structure and regions of the recombinant proteins (marked by the blue line) of human BP180 (A) and BP230 (B) utilized in this enzymelinked immunosorbent assay study. NH2: N-terminus, NC16A: 16th noncollagenous domain, COOH: Cterminus, BP: bullous pemphigoid. |
 | Fig. 2Scatter plot representation of BP180 (NC16A domain, represented in black dots) and BP230 (C- and N-terminal recombinant proteins, represented in red dots) ELISA results (index value). Forty-seven BP sera and 31 control sera (16 EBA sera and 15 healthy volunteer sera) were examined. A dashed line indicates the cut-off value (BP180, 9 index; BP230, 9 index). The arithmetic means of index value are indicated by a line. Index value=(optical density [OD] of tested serum-OD of negative control)/(OD of positive control-OD of negative control)×100. Cut-off values were determined based on the manufacturer's recommendation. BP: bullous pemphigoid, EBA: epidermolysis bullosa aquisita, ELISA: enzyme-linked immunosorbent assay, NC16A: 16th non-collagenous domain. |
 | Fig. 3Correlation between (A) BP180 ELISA scores and disease activity scores, (B) BP230 ELISA scores and disease activity scores, (C) BP180 ELISA scores and blood eosinophil counts, (D) BP230 ELISA scores and blood eosinophil counts in BP patients. BP: bullous pemphigoid, ELISA: enzyme-linked immunosorbent assay. |
 | Fig. 4Comparison of ELISA scores and clinical characteristics of BP patients. (A) BP180 ELISA scores and disease duration, (B) BP230 ELISA scores and disease duration, (C) BP180 ELISA scores and disease severity grade, (D) BP230 ELISA scores and disease severity grade, (E) BP180 ELISA scores and pruritus severity grade, (F) BP230 ELISA scores and pruritus severity grade. BP: bullous pemphigoid, ELISA: enzyme-linked immunosorbent assay. |
 | Fig. 5The index values of BP180 ELISA and BP230 ELISA and titers of indirect immunofluorescence (IIF) in five BP patients before treatment and after remission. BP: bullous pemphigoid, ELISA: enzyme-linked immunosorbent assay. |
 | Fig. 6(A, B) The index values of BP180 ELISA and BP230 ELISA, titers of indirect immunofluorescence (IIF) and disease activity in one BP patient along the entire disease course. BP: bullous pemphigoid, ELISA: enzyme-linked immunosorbent assay. |
References
3. Diaz LA, Ratrie H 3rd, Saunders WS, Futamura S, Squiquera HL, Anhalt GJ, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest. 1990. 86:1088–1094.

4. Stanley JR, Tanaka T, Mueller S, Klaus-Kovtun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients' autoantibodies. J Clin Invest. 1988. 82:1864–1870.

5. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992. 99:243–250.

6. Hirako Y, Usukura J, Nishizawa Y, Owaribe K. Demonstration of the molecular shape of BP180, a 180-kDa bullous pemphigoid antigen and its potential for trimer formation. J Biol Chem. 1996. 271:13739–13745.

7. Schäcke H, Schumann H, Hammami-Hauasli N, Raghunath M, Bruckner-Tuderman L. Two forms of collagen XVII in keratinocytes. A full-length transmembrane protein and a soluble ectodomain. J Biol Chem. 1998. 273:25937–25943.
8. Hopkinson SB, Baker SE, Jones JC. Molecular genetic studies of a human epidermal autoantigen (the 180-kD bullous pemphigoid antigen/BP180): identification of functionally important sequences within the BP180 molecule and evidence for an interaction between BP180 and alpha 6 integrin. J Cell Biol. 1995. 130:117–125.

9. Borradori L, Koch PJ, Niessen CM, Erkeland S, van Leusden MR, Sonnenberg A. The localization of bullous pemphigoid antigen 180 (BP180) in hemidesmosomes is mediated by its cytoplasmic domain and seems to be regulated by the beta4 integrin subunit. J Cell Biol. 1997. 136:1333–1347.

10. Zillikens D, Rose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, et al. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol. 1997. 109:573–579.

11. Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. J Immunol. 1993. 151:5742–5750.

12. Murakami H, Nishioka S, Setterfield J, Bhogal BS, Black MM, Zillikens D, et al. Analysis of antigens targeted by circulating IgG and IgA autoantibodies in 50 patients with cicatricial pemphigoid. J Dermatol Sci. 1998. 17:39–44.

13. Balding SD, Prost C, Diaz LA, Bernard P, Bedane C, Aberdam D, et al. Cicatricial pemphigoid autoantibodies react with multiple sites on the BP180 extracellular domain. J Invest Dermatol. 1996. 106:141–146.

14. Sitaru C, Powell J, Messer G, Bröcker EB, Wojnarowska F, Zillikens D. Immunoblotting and enzyme-linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol. 2004. 103:757–763.

15. Ruhrberg C, Watt FM. The plakin family: versatile organizers of cytoskeletal architecture. Curr Opin Genet Dev. 1997. 7:392–397.

16. Green KJ, Virata ML, Elgart GW, Stanley JR, Parry DA. Comparative structural analysis of desmoplakin, bullous pemphigoid antigen and plectin: members of a new gene family involved in organization of intermediate filaments. Int J Biol Macromol. 1992. 14:145–153.

17. Yang Y, Dowling J, Yu QC, Kouklis P, Cleveland DW, Fuchs E. An essential cytoskeletal linker protein connecting actin microfilaments to intermediate filaments. Cell. 1996. 86:655–665.

18. Hopkinson SB, Jones JC. The N terminus of the transmembrane protein BP180 interacts with the N-terminal domain of BP230, thereby mediating keratin cytoskeleton anchorage to the cell surface at the site of the hemidesmosome. Mol Biol Cell. 2000. 11:277–286.

19. Sonnenberg A, Nievers M, Schaapveld R, Geerts D, Niessen C, Borradori L. Interaction of BP180 and alpha6beta4. J Invest Dermatol. 1999. 112:830–832.
20. Anhalt GJ, Kim SC, Stanley JR, Korman NJ, Jabs DA, Kory M, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990. 323:1729–1735.

21. Hamada T, Nagata Y, Tomita M, Salmhofer W, Hashimoto T. Bullous pemphigoid sera react specifically with various domains of BP230, most frequently with C-terminal domain, by immunoblot analyses using bacterial recombinant proteins covering the entire molecule. Exp Dermatol. 2001. 10:256–263.

22. Kelly SE, Bhogal BS, Wojnarowska F, Whitehead P, Leigh IM, Black MM. Western blot analysis of the antigen in pemphigoid gestationis. Br J Dermatol. 1990. 122:445–449.

23. Zillikens D, Mascaro JM, Rose PA, Liu Z, Ewing SM, Caux F, et al. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol. 1997. 109:679–683.

24. Kobayashi M, Amagai M, Kuroda-Kinoshita K, Hashimoto T, Shirakata Y, Hashimoto K, et al. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci. 2002. 30:224–232.

25. Hofmann S, Thoma-Uszynski S, Hunziker T, Bernard P, Koebnick C, Stauber A, et al. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol. 2002. 119:1065–1073.

26. Nakatani C, Muramatsu T, Shirai T. Immunoreactivity of bullous pemphigoid (BP) autoantibodies against the NC16A and C-terminal domains of the 180 kDa BP antigen (BP180): immunoblot analysis and enzyme-linked immunosorbent assay using BP180 recombinant proteins. Br J Dermatol. 1998. 139:365–370.

27. Mariotti F, Grosso F, Terracina M, Ruffelli M, Cordiali-Fei P, Sera F, et al. Development of a novel ELISA system for detection of anti-BP180 IgG and characterization of autoantibody profile in bullous pemphigoid patients. Br J Dermatol. 2004. 151:1004–1010.

28. Tampoia M, Lattanzi V, Zucano A, Villalta D, Filotico R, Fontana A, et al. Evaluation of a new ELISA assay for detection of BP230 autoantibodies in bullous pemphigoid. Ann N Y Acad Sci. 2009. 1173:15–20.

29. Schmidt E, Obe K, Bröcker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000. 136:174–178.

30. Haase C, Büdinger L, Borradori L, Yee C, Merk HF, Yancey K, et al. Detection of IgG autoantibodies in the sera of patients with bullous and gestational pemphigoid: ELISA studies utilizing a baculovirus-encoded form of bullous pemphigoid antigen 2. J Invest Dermatol. 1998. 110:282–286.

31. Amo Y, Ohkawa T, Tatsuta M, Hamada Y, Fujimura T, Katsuoka K, et al. Clinical significance of enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Dermatol Sci. 2001. 26:14–18.

32. Di Zenzo G, Thoma-Uszynski S, Fontao L, Calabresi V, Hofmann SC, Hellmark T, et al. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol. 2008. 128:415–426.

33. Yoshida M, Hamada T, Amagai M, Hashimoto K, Uehara R, Yamaguchi K, et al. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. J Dermatol Sci. 2006. 41:21–30.

34. Kromminga A, Sitaru C, Hagel C, Herzog S, Zillikens D. Development of an ELISA for the detection of autoantibodies to BP230. Clin Immunol. 2004. 111:146–152.

35. Thoma-Uszynski S, Uter W, Schwietzke S, Hofmann SC, Hunziker T, Bernard P, et al. BP230- and BP180-specific auto-antibodies in bullous pemphigoid. J Invest Dermatol. 2004. 122:1413–1422.

36. Herbst A, Bystryn JC. Patterns of remission in pemphigus vulgaris. J Am Acad Dermatol. 2000. 42:422–427.

37. Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993. 92:2480–2488.

38. Jordon RE, Heine KG, Tappeiner G, Bushkell LL, Provost TT. The immunopathology of herpes gestationis. Immunofluorescence studies and characterization of "HG factor". J Clin Invest. 1976. 57:1426–1431.

39. Korman NJ. In situ-bound antibodies eluted from the skin of patients with bullous pemphigoid are preferentially directed against the 230-kD bullous pemphigoid antigen. J Invest Dermatol. 1995. 105:824–830.

40. Hall RP 3rd, Murray JC, McCord MM, Rico MJ, Streilein RD. Rabbits immunized with a peptide encoded for by the 230-kD bullous pemphigoid antigen cDNA develop an enhanced inflammatory response to UVB irradiation: a potential animal model for bullous pemphigoid. J Invest Dermatol. 1993. 101:9–14.

41. Kiss M, Husz S, Jánossy T, Marczinovits I, Molnár J, Korom I, et al. Experimental bullous pemphigoid generated in mice with an antigenic epitope of the human hemidesmosomal protein BP230. J Autoimmun. 2005. 24:1–10.

42. Tsuji-Abe Y, Akiyama M, Yamanaka Y, Kikuchi T, Sato-Matsumura KC, Shimizu H. Correlation of clinical severity and ELISA indices for the NC16A domain of BP180 measured using BP180 ELISA kit in bullous pemphigoid. J Dermatol Sci. 2005. 37:145–149.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download