Abstract
Background
Skin pigmentary changes of pityriasis versicolor may occur as either hyperpigmented or hypopigmented lesions, depending on the outcome of interactions between Malassezia yeasts and the skin, such as lipoperoxidation process, stimulus of inflammatory cell to melanocytes, and increased thickness of keratin layer.
Objective
To investigate skin characteristic factors that enhance the susceptibility to Malassezia yeasts and provoke different color changes of pityriasis versicolor patients.
Methods
To clarify these factors, we investigated the skin characteristics of pityriasis versicolor patients, using a non-invasive method known as MPA 5® (Courage and Khazaka, Germany). A total of 90 normal healthy subjects and 30 pityriasis versicolor patients were included in this study.
Results
Both hyperpigmented and hypopigmented pityriasis versicolor skin lesions showed higher humidity, increased sebum excretion rate and increased transepidermal water loss (TEWL) values than normal healthy subjects. But no significant difference of specific Malassezia yeasts species between hyperpigmented and hypopigmented skin lesions was evident.
Conclusion
These results indicate that higher humidity and increased sebum level provide a better growing environment of Malassezia yeasts in the skin, leading to the assumption that interaction between Malassezia yeasts and skin barrier materials makes disruption of skin barrier causing increased TEWL.
Pityriasis versicolor is one of the most common disorders of pigmentation in the world. It is a cutaneous, superficial fungal infection characterized by skin pigmentary changes due to colonization of the stratum corneum by a lipophilic fungus in the normal flora of the skin, known as Malassezia furfur and M. globosa1-3. These Malassezia yeasts, discovered in 75~80% of healthy subjects, can cause pityriasis versicolor when the yeast converts to its mycelial form when disposed to certain predisposing factors, such as heat, moisture, occlusion, depressed cellular immunity status, and etc.4,5. Also, it has been suggested that skin pigmentary changes of pityriasis versicolor can occur as either hyperpigmented or hypopigmented lesions in accordance with interactions between Malassezia yeasts and skin characteristics, such as lipoperoxidation process, stimulus of inflammatory cell to melanocytes, and increased thickness of keratin layer6-8. However, the precise factors that enhance susceptibility to Malassezia yeasts and provoke pityriasis versicolor have yet to be completely defined.
To define these factors, we investigated skin characteristics of pityriasis versicolor patients against healthy subjects via non-invasive method, known as MPA 5®9 in this study. MPA5 is a non-invasive physiological parameter of the skin for evaluating the levels of sebum, skin hydration, transepidermal water loss (TEWL) and skin complexion. Although MPA 5® does have some advantages, such as non-invasiveness and faster detection of skin characteristics applicable to large subjects, it shows large variations depending on the specific body sites, temperature, humidity of measuring sites, and age, sex, and etc.10-14. Taking that into account, we established the normal range values of 90 healthy subjects in specific condition, and then compared the skin characteristics of pityriasis versicolor patients in the same condition before further proceeding with the study. Moreover, to investigate the correlation between specific Malassezia yeast and skin color changes in pityriasis versicolor, molecular biological evaluation of the skin lesion was carried out.
This study was approved by the ethnics committee of the Institutional Review Board.
The study group was composed of 63 men and 27 women of normal healthy subjects and 30 patients (21 males and 9 females) with pityriasis versicolor, who were diagnosed at the Department of Dermatology, Konkuk University Hospital from March 2008 through February 2009. The diagnosis of pityriasis versicolor was made by a positive KOH examination, showing typical hyperpigmented or hypopigmented scaly patches. History of treatment and duration of disease were not considered. Average age of normal healthy subjects was 38±11.8, and that of patients group was 32±4.4. Among the patients group, 21 patients had hyperpigmented skin lesions and 9 hypopigmented skin lesions.
All subjects washed off the areas to be measured with cleanser in the outpatient clinic, and was allowed to be assimilated to the conditions of constant temperature and humidity (Room humidity and room temperature 38~42%, 23~25℃) for 20 minutes before the measurement.
Skin lipid content was measured using Sebumeter® SM 815 (Courage and Khazaka, Cologne, Germany).
Skin water content was measured by Corneometer® CM 825 (Courage and Khazaka), and the capacitance of the area of the skin with the highest coefficient of insulation was chosen for measurement, thus estimating the relative amount of water content. The average of the three measurements for each area was selected.
The amount of water evaporated from the skin surface was measured with Tewameter® TM 300 (Courage and Khazaka), and the value reflects the proportion of water vapor evaporating from the skin surface, per given unit of surface area (g/m2h) and time. Measurement was taken for one time for each area.
Sterile cotton swabs were moistened with wash fluid containing 0.1% Triton X-100 in 0.075 M phosphate buffer (pH 7.9), and rubbed gently, with rotation. Swabbing was performed for 10 seconds on the skin lesion of pityriasis versicolor. Swabs were immediately placed in Leeming and Notman agar media and incubated at 34℃ for 14 days.
The yeasts grown in agar were harvested, and resuspended in 0.4 ml of lysis buffer (100 mM Tris-HCl pH 8.0, 1.0% SDS, 2.0% Triton X-100, 10 mM EDTA, 100 mM NaCl). Equal volumes of phenol/chloroform/isoamyl alcohol (phenol:chloroform:isoamyl alcohol=25:24:1, v/v) and glass beads (0.5 mm) were added, and the mixture was vortexed for 10 minutes. The samples were centrifuged at 12,000×g for 15 minutes. Total DNA was precipitated from the aqueous fraction with isopropranol and centrifuged at 12,000×g for 20 minutes at 4℃. The DNA pellet was washed in 70% ethanol and resuspended in sterile water.
To amplify 26S rDNA from genomic DNA, reaction mixture contained 25 mM of each dNTP, 10X PCR buffer, 5X Q buffer, 0.5 µM primer, 0.4 µm forward primer (5'-TAACAAGGATTCCCCTAGTA-3', reverse primer (5'-ATTACGCCAGCATCCTAAG-3'), and 1.25 U Hot StarTaq polymerase in 50 µl reaction volume. 35 cycles with the following profile were programmed: denaturation for 45 seconds at 94℃; annealing for 45 seconds 52.5℃; extension for 1 minute at 72℃. After the confirmation of the amplified 26S rDNA, PCR (Veriti® 96-Well Fast Thermal Cycler. ABI) products were purified, using a LaboPass™ Gel (Cosmo, Seoul, Korea) and PCR Clean-up kit (Cosmo). Two restriction enzymes, Hha I (Koschem, Seoul, Korea) and BtsC I (NEB, London, England) were used to perform 26S rDNA-RFLP of Malassezia. In this experiment, the restriction enzyme digestion was performed with 10×PCR buffer, 10 U of the restriction enzyme, and PCR products 7.5 µl, which sum up to 20 µl. After the reaction at 37℃ for 3 hours, the electrophoresis was done with 3% (w/v) Seakem LE agarose gel (Takara Biomedicals, Otsu, Japan) by 100 volt and stained with ethidium bromide. Restriction fragments were analyzed with the size and number of DNA fragments under the UV transilluminator.
The measured values from the patient and control groups were compared and analyzed using SPSS ver 12.0 (SPSS Inc., Chicago, IL, USA). Comparative analysis of the interspecies differences and the experimentally derived values were carried out using independent samples t-test. For testing of statistical significance among the sites measurement, ANOVA test was used.
A total of 90 healthy subjects without skin diseases (63 men [36±12.9] and 27 women [34±12.1] in their age 10~50) were included in this study, and the results obtained by non-invasive bioengineering method is as follows.
In the case of the cheek, skin lipid content was 64.2±8.6 in men, and 46.8±8.9 in women; and in the case of trunk, 62.9±22.1 in men and 43.2±19.6 in women; and in the case of the dorsum of hand, 18.7±6.3 in men and 16.0±4.7 in women. The values were significantly higher in men at the cheek and the trunk (p<0.05), and they were lower in the dorsum of hand, but not statistically significant (p>0.05). By the site, the lipid content was the highest in the cheek, followed by the trunk, and the dorsum of hand both in men and women (p<0.05) (Table 1).
Skin water content was 58.3±11.9 in men and 45.2±16.5 in the case of cheek, and 52.0±19.7 in men and 40.6±20.9 in women in the case of the trunk, and in the dorsum of hand 49.3±18.2 in men and 51.1±16.4 in women, and the values were significantly higher in men compared to women in all body sites, except the dorsum of hand (p<0.05). By the body site, the values were highest in the cheek followed by the trunk and dorsum of hand in men, whereas in women, it was the highest in the dorsum of hand followed by the cheek and trunk (Table 1).
TEWL was 15.3±4.2 in men and 13.2±3.8 in women in the case of the cheek, 14.6±7.9 in men and 11.4±5.1 in the case of the trunk, and 11.9±6.9 in men and 8.2±1.5 in women in the case of dorsum of hand. The values were higher in men, but not statistically significant (p>0.05). By the body site, the value was highest in the cheek, followed by the trunk and the dorsum of hand in both men and women (p<0.05) (Table 1).
MI and EI as measured by Mexameter® were 244.3±54.1/342.7±64.9 in men and 228.3±30.8/329.3±78.1 in women in the case of cheek, 224.1±58.0/320.7±83.4 in men and 211.6±82.0/304.5±106.4 in women in the case of trunk and 278.3±55.7/315.3±38.5 in men and 256.8±42.3/305.0±66.1 in women in the case of dorsum of hand. The values were higher in men in both melanin and erythema indices (p<0.05). By the body site, MI was highest in the dorsum of hand, followed by the cheek and the trunk in both men and women. In the case of EI, the values were highest in the cheek, followed by the trunk and dorsum of hand in men, and in women, they were highest in the cheek followed by the dorsum of hand and trunk (p<0.05) (Table 1). The measured values were higher in men in all body sites, a finding that was statistically significant (p<0.05). By the body site, the values were highest in the cheek, followed by the trunk and dorsum of hand in men, and in women, they were highest in the dorsum of hand followed by the cheek and trunk (Table 1).
The age ranged varied from the teens to sixties, and on comparing the average values of the patient group to the controls, the TEWL values were highest in the fifties, and in other indices, in the sixties.
In the case of pityriasis versicolor patients group, age and sex matched 30 patients from their teens to their fifties (10~50 age [36±14.0]), who developed skin lesions in weeks to months before, hyperpigmented lesions were observed in 21 patients (38±14.2), and hypopigmented lesions in 9 patients (31±13.0) (Fig. 1). The results of comparison of the two clinical manifestations are as follows;
Skin lipid content in pityriasis versicolor patients with hyperpigmented lesion was 204.5±77.3 in men and 177.4±41.7 in women, and in hypopigmented lesion 211.7±81.5 in men and 177.8±40.1 in women, which was statistically significant in both types of lesions in the same sex group (p<0.05). In both types of lesions, the lipid content was higher in men (p<0.05) (Fig. 2), and the values were slightly higher in the hypopigmented lesion, which was not statistically significant (Table 2).
Skin water content in pityriasis versicolor patients with hyperpigmented lesion was 71.3±16.4 in men and 63.4±19.3 in women, and in the case of hypopigmented lesion, 62.8±16.9 in men and 50.5±19.9 in women, which was not a statistically significant increase in both types of lesions (p>0.05) (Table 2). The values were higher in the patient group in both types of lesions, but not statistically significant (Fig. 2).
TEWL values in pityriasis versicolor patients with hyperpigmented lesion were 28.4±7.0 in men and 24.0±4.6 in women, and in the case of hypopigmented lesion, 24.1±6.2 in men and 21.7±6.7 in women. In both types of lesions, the values were higher in men but not statistically significant (p>0.05) (Table 2). The values were higher in the patient group in both types of lesions, a finding that was not statistically significant (p<0.05) (Table 2).
Melanin and erythema indices as measured by Mexameter® in pityriasis patients with hyperpigmented lesion was 318.5±41.2/474.6±70.2 in men and 307.4±32.1/451.8±71.1 in women, and in the case of hypopigmented lesion, 206.7±33.2/312.0±54.3 in men and 200.9±31.9/305.6±54.1 in women, a finding that was not statistically significant (p>0.05) (Table 2). Also, there was statistically significant increase in the case of hyperpigmented lesions only in the patient group compared to the control group (p<0.05) (Fig. 2).
On RFLP performed to identify the difference between the incidences of species of Malassezia yeasts in pityriasis versicolor patients with hyperpigmented and hypopigmented lesions, there was no significant difference and five species, i.e., M. globosa, M. restricta, M. furfur, M. sympodialis, and M. Slooffiae were isolated (Fig. 3, 4). Among these, M. globosa was most frequently isolated in all body sites, followed by M. restricta, M. furfur, M. slooffiae, and M. sympodialis (Table 3).
Malassezia yeasts are normal flora of the skin that are discovered in 75~80% of health subjects, and are classified into 7 species, M. furfur, M. pachydermatis, M. sympodialis, M. globosa, M. obtusa, M. restricta and M. slooffiae in 1996 by Guého et al.15. Recently, on the basis of DNA-relatedness, through the molecular biology, four new species have been included: M. dermatis, M. japonica, M. nana and M. yamatoensis16-21. In Europe, M. caprae and M. equina were additionally isolated, and now Malassezia yeasts are classified into 13 species22. Among these Malassezia yeasts, M. furfur has long been identified as the fungus causing pityriasis versicolor, and recently it was known that M. globosa was also associated with a vast majority of lesions3,23,24.
The specific condition that opportunistic Malassezia yeasts induce pityriasis versicolor was reported as exogenous and endogenous factors. The exogenous factors include heat and moisture, a probable reason why the disease is more prevalent in the tropics and in the summer in more temperate climates. Another exogenous factor may be occlusion of the skin by either clothing or cosmetics2. Occlusion results in increased carbon dioxide concentration25, an altered microflora and pH range. Infection has been experimentally induced by an occlusive dressing26. Otherwise, endogenous factors account for the prevalence of the disease in temperate climates. Seborrheic dermatitis, Cushing's syndrome, immunosuppressive treatment (depressed cellular immunity), malnutrition, and hyperhidrosis (particularly flexural) have all been implicated as endogenous factors27-29.
Clinical manifestation of pityriasis versicolor is scaly hypopigmented or hyperpigmented macules observed in characteristic areas of the body, including the chest, back, abdomen, and proximal extremities4,5. The variation in pigmentation, despite being the same lesion, can be explained by the interaction between skin barrier components and Malassezia yeasts, such as lipoperoxidation process causing cytotoxic effect to make hypopigmented patches, increased keratin layer thickness or stimulation of melanocytes by inflammatory cells to make hyperpigmented patches6-8.
Noninvasive methods as the means of assessing the characteristics according to sex, age and body site are being explored by many investigators. Noninvasive methods are more objective than gross inspection and the measurement is handy and more valuable in that it leaves the skin intact9,30.
In this study, to evaluate the differences of skin characteristics between pityriasis versicolor patients and normal healthy subjects, we investigate the degree of water preservation, according to sex and age in healthy adults (Corneometer®), TEWL (Tewameter®), sebum excretion rate (Sebumeter®), MI and EI (Mexameter®) using non-invasive bioengineering methods, known as MPA 510-14.
Previous studies by Koh et al.9, Conti et al.31 measuring the physiologic factors of the skin in healthy adults differed from each other, according to sex, age and the site of measurement. In this study, the lipid content was higher in men in the body sites, such as the cheek and trunk in healthy controls, a finding that is consistent with the results obtained by Conti et al.31 and also the values of skin water content and TEWL values were higher in men, which was consistent with the results obtained by Conti et al.31. Moreover, the melanin and erythema indices as measured by Mexameter® were higher in men in all body sites, which is in agreement with results obtained by Koh et al.9.
In this study the sites of measurement in 30 pityriasis versicolor patients with hypopigmented and hyperpigmented lesions were the cheek, chest, abdomen and the back, and on measuring the values on the corresponding areas in the controls, the capacitance, reflecting the degree of water preservation, was not significantly different, but in the case of hyperpigmented lesion, TEWL, sebum excretion rate, MI & EI values differed significantly7,15,32, and in the case of hypopigmented lesion, there were statistically significant differences in TEWL and sebum excretion rate. Also, all the values were higher in the patient group, except MI and EI of pityriasis versicolor patients with hypopigmented lesions6,8.
Our results show that pityriasis versicolor skin lesion has higher humid status, higher sebum excretion rate and increased TEWL values than those of normal healthy subjects. And, there was no significant differences between hyperpigmented and hypopigmented skin lesion. These results indicate that higher humidity and sebum excretion of the skin will makes well growing status of Malassezia yeasts, and presume that interaction between Malassezia yeasts and skin barrier materials makes disruption of skin barrier causing increased TEWL. Therefore, hydration and sebum excretion rate are cause of pityriasis versicolor, and TEWL is the result of pityriasis versicolor. Moreover, given the increase in TEWL in both hyperpigmented and hypopigmented lesions, it can be thought that hyperpigmented lesions are induced by the stimulation of melanocytes by inflammatory cells, rather than increase in the thickness of the keratin layer6-8,33.
Also, on RFLP performed to identify the difference between the species of Malassezia yeasts in pityriasis versicolor patients with hyperpigmented and hypopigmented lesions, there was no significant difference and five species, i.e., M. globosa, M. restricta, M. furfur, M. sympodialis, and M. Slooffiae were isolated (Fig. 3, 4). Among these, M. globosa was most frequently isolated in all body sites, followed by M. restricta, M. furfur, M. sympodialis, and M. slooffiae. The findings did not differ significantly from previous studies on isolation of the types of Malassezia yeasts from the lesions of pityriasis versicolor24,33,34.
In turn, the species of Malassezia yeasts has not a decisive effect on the difference in skin color between hyperpigmented and hypopigmented lesions, but according to the reported literatures, the interactions between Malassezia yeasts and skin barrier materials, which makes lipoperoxidation causing hypopigmentation or stimulation of melanocytes by inflammatory cells causing hyperpigmentation. Our limitations in this study include the fact that we had a relatively low population of pityriasis versicolor patients, compared to the normal healthy subjects. Further, it is needed that the additional studies of the interactions between Malassezia yeasts and skin barrier materials, which make lipoperoxidation or stimulation of melanocytes. Future additional studies will hopefully be successful in ascertaining these differences and exact value ranges of MPA 5®.
Figures and Tables
Fig. 1
(A, C) Hyperpigmented lesions of two representative patients on the back and anterior chest. (B, D) Hypopigmented lesions of two representative patients on the cheek and posterior neck.
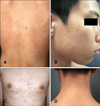
Fig. 2
(A) Comparison of TEWL values, (B) sebum excretion rate, (C) capacitance values, (D) melanin index and (E) erythema index between healthy control and patients. TEWL: transepidermal water loss, M: male, F: female. t-test paired comparison were performed versus healthy control; the *shows statistically significant differences at p<0.05.

Fig. 3
PCR-RFLP patterns of 26S rDNA PCR digested with restriction enzymes (A) Hha I, (B) BtsC I of 11 Malassezia standard strains in hyperpigmented lesions of 21 patients. Lanes: M: molecular Marker, 1: Malassezia globosa (CBS7966), 2: M. globosa (CBS7966), 3: M. restricta (KCTC7848), 4: M. globosa (CBS7966), 5: M. restricta (KCTC7848), 6: M. restricta (KCTC7848), 7: M. restricta (KCTC7848), 8: M. slooffiae (KCTC17431), 9: M. globosa (CBS7966), 10: M. globosa (CBS7966), 11: M. restricta (KCTC7848), 12: M. globosa (CBS7966), 13: M. globosa (CBS7966), 14: M. furfur (KCTC7743), 15: M. slooffiae (KCTC17431), 16: M. globosa (CBS7966), 17: M. globosa (CBS7966), 18: M. restricta (KCTC7848), 19: M. restricta (KCTC7848), 20: M. furfur (KCTC7743), 21: M. sympodialis (KCTC7985). PCR: polymerase chain reaction, RFLP: restriction fragment length polymorphism.

Fig. 4
PCR-RFLP patterns of 26S rDNA PCR digested with restriction enzymes (A) Hha I, (B) BtsC I of 11 Malassezia standard strains in hypopigmented lesions of 9 patients. Lanes: M: molecular Marker, 1: Malassezia slooffiae (KCTC17431), 2: M. globosa (CBS7966), 3: M. restricta (KCTC7848), 4: M. restricta (KCTC7848), 5: M. globosa (CBS7966), 6: M. globosa (CBS7966), 7: M. globosa (CBS7966), 8: M. sympodialis (KCTC7985), 9: M. furfur (KCTC7743). PCR: polymerase chain reaction, RFLP: restriction fragment length polymorphism.

Table 1
Casual level transepidermal water loss values (g/m2h), sebum excretion rate, capacitance values, melanin and erythema index in relation to site and sex (mean±standard deviation) - healthy subjects

Table 2
Casual level of transepidermal water loss values (g/m2h), sebum excretion rate, capacitance values, melanin and erythema index in relation to site and sex (mean±standard deviation) - pityriasis versicolor patients

All numerical values are arbitrary unit. Significance versus sex by independence samples t-test and significance versus sites by ANOVA test. TEWL: transepidermal water loss. *Significant differences between healthy control and patient, p<0.05, †significant differences between male and female, p<0.05.
References
1. Adamski Z. Studies of a role played by lipophilic yeasts Malassezia furfur (Pityrosporum ovale, Pityrosporum orbiculare) in different dermatoses. Postepy Dermatol (Poznan). 1995. 12:349–454.
3. Morishita N, Sei Y, Sugita T. Molecular analysis of malassezia microflora from patients with pityriasis versicolor. Mycopathologia. 2006. 161:61–65.

4. Ahn KJ. Malassezia species cultured from the lesions of pityriasis versicolor. Korean J Dermatol. 1997. 35:736–743.
5. Ahn KJ. Taxonomy of the genus Malassezia. Korean J Med Mycol. 1998. 3:81–88.
6. Yoo DW, Kim HJ, Kim YW, Ro BI, Chang CY. Electron microscopic study in tinea versicolor: structural changes of melanosomes accompanying the hyperpigmented and hypopigmented lesions. Korean J Dermatol. 1983. 21:63–70.
7. Allen HB, Charles CR, Johnson BL. Hyperpigmented tinea versicolor. Arch Dermatol. 1976. 112:1110–1112.

8. Charles CR, Sire DJ, Johnson BL, Beidler JG. Hypopigmentation in tinea versicolor: a histochemical and electronmicroscopic study. Int J Dermatol. 1973. 12:48–58.

9. Koh JS, Chae KS, Kim HO. Skin characteristics of normal Korean subjects according to sex and site using non-invasive bioengineering methods. Korean J Dermatol. 1998. 36:855–864.
10. Serup J, Jemec GBE. Handbook of non-invasive methods and the skin. 1995. Boca Raton: CRC Press;3–8.
11. Moss J. The effect of 3 moistures on skin surface hydration. Skin Res Technol. 1996. 2:32–36.
12. Thoma S, Welzel J, Wilhelm KP. Relationship between transepidermal water loss and temperature of measuring probe. Skin Res Technol. 1997. 3:73–80.

13. Cunliffe WJ, Kearney JN, Simpson NB. A modified photometric technique for measuring sebum excretion rate. J Invest Dermatol. 1980. 75:394–398.

14. Jang HY, Park CW, Lee CH. A study of transepidermal water loss at various anatomical sites of the skin. Korean J Dermatol. 1996. 34:402–406.
15. Guého E, Midgley G, Guillot J. The genus Malassezia with description of four new species. Antonie Van Leeuwenhoek. 1996. 69:337–355.

16. Sugita T, Kodama M, Saito M, Ito T, Kato Y, Tsuboi R, et al. Sequence diversity of the intergenic spacer region of the rRNA gene of Malassezia globosa colonizing the skin of patients with atopic dermatitis and healthy individuals. J Clin Microbiol. 2003. 41:3022–3027.

17. Sugita T, Takashima M, Shinoda T, Suto H, Unno T, Tsuboi R, et al. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J Clin Microbiol. 2002. 40:1363–1367.

18. Sugita T, Takashima M, Kodama M, Tsuboi R, Nishikawa A. Description of a new yeast species, Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. J Clin Microbiol. 2003. 41:4695–4699.

19. Hirai A, Kano R, Makimura K, Duarte ER, Hamdan JS, Lachance MA, et al. Malassezia nana sp. nov., a novel lipid-dependent yeast species isolated from animals. Int J Syst Evol Microbiol. 2004. 54:623–627.

20. Sugita T, Tajima M, Takashima M, Amaya M, Saito M, Tsuboi R, et al. A new yeast, Malassezia yamatoensis, isolated from a patient with seborrheic dermatitis, and its distribution in patients and healthy subjects. Microbiol Immunol. 2004. 48:579–583.

21. Lee YW, Yim SM, Lim SH, Choe YB, Ahn KJ. Quantitative investigation on the distribution of Malassezia species on healthy human skin in Korea. Mycoses. 2006. 49:405–410.

22. Cabañes FJ, Theelen B, Castellá G, Boekhout T. Two new lipid-dependent Malassezia species from domestic animals. FEMS Yeast Res. 2007. 7:1064–1076.
23. Gandra RF, Simão RC, Matsumoto FE, da Silva BC, Ruiz LS, da Silva EG, et al. Genotyping by RAPD-PCR analyses of Malassezia furfur strains from pityriasis versicolor and seborrhoeic dermatitis patients. Mycopathologia. 2006. 162:273–280.

24. Gaitanis G, Velegraki A, Alexopoulos EC, Chasapi V, Tsigonia A, Katsambas A. Distribution of Malassezia species in pityriasis versicolor and seborrhoeic dermatitis in Greece. Typing of the major pityriasis versicolor isolate M. globosa. Br J Dermatol. 2006. 154:854–859.

25. King RD, Cunico RL, Maibach HI, Greenberg JH, West ML, Jeppsen JC. The effect of occlusion on carbon dioxide emission from human skin. Acta Derm Venereol. 1978. 58:135–138.
26. Faergemann J, Bernander S. Tinea versicolor and Pityrosporum orbiculare: a mycological investigation. Sabouraudia. 1979. 17:171–179.
27. Congly H. Pityriasis versicolor in a 3-month-old boy. Can Med Assoc J. 1984. 130:844–845.
28. Roberts SO. Pityriasis versicolor: a clinical and mycological investigation. Br J Dermatol. 1969. 81:315–326.

29. Burke RC. Tinea versicolor: susceptibility factors and experimental infection in human beings. J Invest Dermatol. 1961. 36:389–402.

30. Hayakawa R. Measurement methods and evaluation of skin surface lipids. Fragrance J. 1988. 92:26–30.
31. Conti A, Schiavi ME, Seidenari S. Capacitance, transepidermal water loss and causal level of sebum in healthy subjects in relation to site, sex and age. Int J Cosmet Sci. 1995. 17:77–85.

32. Ozawa T, Takahashi M. Skin hydration: recent advances. Acta Derm Venereol Suppl (Stockh). 1994. 185:26–28.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download