Abstract
Background
The pathogenesis of psoriasis may involve the interleukin (IL)-23 and Th17-mediated immune responses. Th17 cells secret IL-17 and IL-22, which mediates dermal inflammation and acanthosis.
Objective
As inhibitor of nuclear factor κB kinase-α (IKKα) has been previously identified as a primary regulator of keratinocyte differentiation and proliferation, we proposed that IL-17 and IL-22 might affect keratinocyte differentiation by changing the expression of IKKα.
Methods
We employed HaCaT cells
maintained culture medium at a low calcium concentration (0.06 mM) and induced differentiation by switching to the high concentration (2.8 mM) media with IL-17 or IL-22, then compared the IKKα expression and the cell cycle. We employed reconstituted human epidermal skin (Neoderm) and mice ears for the in vivo studies.
Results
Elevated
calcium concentration induced IKKα expression and terminal differentiation with cell cycle arrest in HaCaT cell cultures. Moreover, IL-17 and IL-22 treatment also induced IKKα in HaCaT cells and reconstituted human epidermis. IKKα induction was also noted, following the injection of IL-17 and IL-22 into mice ears.
Conclusion
Although the induction of IKKα was accompanied by keratinocyte differentiation, IL-17 and IL-22 did not affect calcium-mediated differentiation or the cell cycle. Rather, IL-17 and IL-22 appear to contribute to the inflammation occurring via the induction of IKKα from keratinocytes or skin layers.
Psoriasis is a chronic skin disease, characterized by the presence of dry red, raised, scaly plaques, which can be several centimeters in diameter. Usually, the skin is covered with many individual lesions, separated by normal appearing skin; whereas, in severe cases, the entirety of the skin may be affected. The most specific histopathological changes distinguishing psoriasis from other inflammatory skin diseases are dramatic hyperplasia of the epidermis with a loss of the granular layer, regular elongation of the rete ridges, thickening of the cornified layer, as well as incomplete keratinocyte differentiation, multiple infiltrations of a variety of leukocytes, and increased vascularity in the dermis1,2. The pathogenesis of psoriasis may involve IL-23 and Th17-mediated immune responses via IL-17 and IL-22; this proposition is based on the characteristic features of psoriatic lesions, which include neutrophil chemotaxis and elevated expression of key antimicrobial peptides3. IL-22 mediates IL-23-induced dermal inflammation and acathosis4. IL-22 induces keratinocyte proliferation and epidermal hyperplasia via the down modulation of terminal keratinocyte differentiation genes3,5.
Keratinocyte differentiation is the process of cellular maturation in the epidermal layers, from the basal cells, spinous cells, granular cells, and cornified cells to build up the skin barrier, in order to protect the body surfaces. Calcium is one of the primary regulators of keratinocyte differentiation. Alterations in the calcium levels have been observed in different epidermal layers6; this finding strongly indicates that calcium may be a key regulator in the induction and maintenance of terminal differentiation status in the epidermal layers7. Additionally, the inhibitor of nuclear factor κB kinase-α (IKKα) has been identified as a primary regulator of keratinocyte differentiation and proliferation. The loss of IKKα prevents terminal differentiation and promotes keratinocyte proliferation, even at the elevated calcium concentrations8,9.
According to the findings of the previous reports, IL-22 and IL-20 play a major role in the pathogenesis of acanthosis and hypogranularity, as compared with IL-17 or IFN-γ. Therefore, we sought to evaluate the relationship between Th17 cytokine and keratinocyte differentiation via IKKα expression.
The HaCaT cells (human keratinocyte cell line) were cultured in DMEM (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco) and 100 U/ml of penicillin/streptomycin (Gibco) at 37℃ in an incubator containing 5% CO2. The cells were grown to 70~80% confluence and stimulated with conditioned medium, containing 200 ng/ml of IFN-γ, IL-17, or IL-22 for 24 hours, then harvested for protein extraction.
C57BL/6 mice were acquired from Daehan Biolink (Eumseong, Korea). The mice were housed and bred under specific pathogen-free conditions at the animal facility of the Ewha Medical School. All animal studies were approved by the Animal Care and Use Committee of Ewha Medical School (ESM 09-0120) and conformed to international standards. In order to evaluate the effects of local administration of IL-17 or IL-22 on mouse skin, we injected a 30µl volume of phosphate-buffered saline (PBS), either alone or containing 3µg of recombinant mouse IL-17A (576004; Biolegend, San Diego, CA, USA) or recombinant mouse IL-22 (576204; Biolegend) intradermally into both ears of each anesthetized mouse, using a 30-gauge needle for the two consecutive days. Twentyfour hours after the final injection, the mouse ears were collected via punch biopsy (6 mm in diameter).
HaCaT cells (5×105 cells/well) were treated for 5 days with 100 ng/ml of IL-17 or IL-22, under low or high calcium concentrations (0.06 and 2.8 mM, respectively). For a cell cycle analysis, the cells were detached with trypsin, and washed twice in cold PBS. The cells were then fixed in 70% ethanol and the cellular DNA was stained by incubating the cells in 500µl of PBS, containing a 50µg/ml of propidium iodide, 5 mM EDTA, and 1 mg/ml of RNase for 30 minutes at room temperature (RT). After staining, the cells were acquired in a FL2-area versus FL2-width plot to exclude the aggregates (doublets) using a FACSCalibur apparatus (BD Biosciences, San Jose, CA, USA) with CellQuest software (BD Biosciences). Cell cycle fitting was conducted using ModFitLT software (Verity Software House, http://www.vsh.com). The proliferation index (PI) was calculated as PI=(S+G2/M)/(G0/G1+S+G2/M).
We purchased Neoderm®-E grown for 12 days at the air-medium interface from TEGO Science (Seoul, Korea [http://www.tegoscience.com]), and cultured the samples at 37℃, 10% CO2, and ambient humidity. We fed the graft by changing the medium every four or five days. For cytokine stimulation, grafts were incubated for 48 hours with 100 ng/ml of IL-17 or IL-22.
Cells were lysed in a lysis buffer (1% Triton X-100, 150 mM NaCl, 20 mM Tris, pH 7.5), containing protease inhibitors, and 10µg of proteins were separated via electrophoresis on 12% SDS-polyacrylamide gels. The resolved proteins were then transferred to polyvinylidene difluoride membranes (Milipore, Bedford, MA, USA). The membranes were blocked with 5% skim milk in Tris-buffered saline and subsequently incubated with the following primary antibodies: anti-involucrin (SY5, mouse monoclonal; Abcam, Cambridge, UK), anti-phospho-IκB-α (B-9, mouse monoclonal; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-IKKα (B-8, mouse monoclonal; Santa Cruz Biotechnology), anti-cytokeratin 5 (goat polyclonal, sc-17090; Santa Cruz Biotechnology), cytokeratin 10 (4A27, mouse monoclonal; Santa Cruz Biotechnology), and anti-β-actin (H11, mouse monoclonal; Applied Biomedical Materials Inc., Richmond, Canada). After washing, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G secondary antibody, and the signals were visualized with an enhanced chemiluminescence ECL kit (Thermo Fisher Scientific Inc., Waltham, MA, USA), in accordance with the manufacturer's instructions.
For histological and immunological staining, Neoderm® grafts and punched mice ears were fixed in 4% formalin solutions for more than 4 hours and paraffin-embedded. The sections were stained with hematoxylin and eosin (H&E stain) or with IKKα-specific primary antibodies, followed by the peroxidase-conjugated secondary antibodies; and then subsequently counterstained with hematoxylin. The sections were deparaffinized via two incubations in xylene and sequential rehydration steps with a graded series of ethanol, followed by washing in water. The tissue sections were subsequently incubated with blocking solution (DakoCytomation, Glostrup, Denmark) for 30 minutes at RT, in order to terminate the endogenous peroxidase activity, followed by 10 minutes of protein blocking (DakoCytomation). After washing the slides with PBS, the sections were incubated for 30 min with a secondary antibody solution (DakoCytomation), washed again, and added to HRP-conjugated streptavidin reagents. After washing, the sections were incubated with 3,3'-diabenzidine (DAB) substrate (DakoCytomation) for color development.
To quantify IL-1β, IL-8 and TNF-α secretion, the culture supernatants of HaCaT cells were collected and the levels of secreted proteins were determined, using an ELISA kit, in accordance with the manufacturer's recommended protocols (BD Bioscience, San Diego, CA, USA). In brief, the wells were coated with 100µl of capture antibody in a coating buffer (0.1 M sodium carbonate, pH 9.5) and the plate was incubated overnight at 4℃. After washing the wells with a washing buffer (PBS with 0.05% Tween-20), the wells were blocked with assay diluents (10% fetal bovine serum in PBS) for 1 hour at RT, followed by the addition of 100µl/ well of the cell supernatant and cytokine standard solutions for 2 hours at RT. After washing, 100µl of detection antibody and streptavidin-conjugated horseradish peroxidase (HRP) reagent were added to the wells, and incubated for 1 hour at RT. After extensive washing, 100µl of substrate solution (tetramethylbenzidine and hydrogen peroxide, BD Biosciences) was added to each well and the plates were incubated for 30 minutes at RT in the dark. Stop solutions (2 N H2SO4) were added and absorbance at 450 nm was read within 30 minutes. The ELISA detection limits were as follows: 3.9 pg/ml for IL-1b, 3.1 pg/ml for IL-8, and 7.8 pg/ml for TNF-α. For observing the effect of IL-17 and IL-22 in HaCaT cells, controlled by nuclear factor kappa B (NF-κ B) on cytokine secretion, we used PS-1145 (Sigma), an inhibitor of IKK that is the upstream activator of NF-κB, at a concentration of 10 µM with pre-incubation of the cells for an hour.
To induce HaCaT differentiation, we employed the established keratinocyte differentiation model10 by switching the calcium concentration from low (0.06 mM) to high (2.8 mM). In order to confirm the keratinocyte differentiation, we collected HaCaT cells every 24 hours and evaluated the expression of involucrin as a marker of terminal differentiation, as well as cytokeratin 5 and cytokeratin 10, which are markers of basal and spinous cells11, after switching to a high-calcium concentration medium for up to 120 hours. After 48 hours, the expression of cytokeratin 5 and cytokeratin 10 began to decline, in contrast to the observed increase in IKKα and involucrin levels (Fig. 1). This suggests that IKKα expression is correlated with the keratinocyte differentiation. Moreover, according to these results, we concluded that the elevated calcium concentration affected the level of IKKα, which eventually induced the terminal differentiation in the keratinocytes.
Next, we attempted to analyze and compare the cell cycle between the two different groups of HaCaT cells: those maintained under low-calcium conditions, versus those switched to a high calcium-concentration culture media. In addition, because Th17 cytokines, i.e., IL-17 and IL-22 are involved in the pathogenesis of inflammatory skin disease3, we compared the cell cycle and proliferation indices under the influence of IL-17 and IL-22. To compare the cell cycle characteristics of the HaCaT cells, according to calcium concentration, as well as IL-17 and IL-22 cytokine conditions, we cultured the HaCaT cells with or without 100 ng/ml of IL-17 and IL-22, at calcium concentrations of 0.06 mM or 2.8 mM. We determined that at the high calcium concentrations, more of the HaCaT cells remained in G0/G1 phase, relative to the population in the low calcium media, in which more of HaCaT cells remained in the proliferative phase (S or G2/M phase); particularly, after 4 or 5 days of media switching (Fig. 2). As shown in the PI graph, HaCaT cells cultured in higher concentrations of calcium media evidenced reduced PI values after media exchange. Th17 cytokines, however, did not affect the cell cycle of the HaCaT cells under the same calcium concentration conditions, and no significant differences in PI were detected (Fig. 2). Therefore, we concluded that calcium-dependent cell cycle arrest and differentiation overrode the possible effect induced by the Th17 cytokines, IL-17 and IL-22, on HaCaT cells during calcium-induced differentiation.
We then postulated that the effect of Th17 cytokines might be observed in HaCaT cells after terminal differentiation. Therefore, we employed HaCaT cells to be maintained at a calcium concentration of 2.8 mM, followed by 24 hours of treatment with 100 ng/ml of IL-17 or IL-22, and the protein extracts were prepared for the immunoblotting assay. Both IL-17- and IL-22-treated groups exhibited a significant increase in the IKKα expression, and phosphorylated IκB expression was also increased significantly via the addition of IL-17 or IL-22 (Fig. 3).
In order to confirm that IKK involved the pro-inflammatory cytokine secretion from HaCaT cells, we used IKK inhibitor, PS1145 (Fig. 4)12. We found that under the PS1145 treatment, the concentration of IL-8 and TNF-α was decreased with IL-17 or IL-22 treatment. Next, we treated the artificial skin layer and the mouse ears with IL-17 or IL-22, and compared the levels of IKKα expression. Neoderm was cultured for 48 hours with 100 ng/ml of IL-17 or IL-22 and fixed for tissue staining. We noticed that IL-17 and IL-22 both induced an increase in IKKα (Fig. 5). These findings suggest that Th17 cytokines elevated the expression levels of IKKα in calcium-mediated differentiated HaCaT cells (2.8 mM maintained cells) or artificial human epidermal layers, which were bathed in 2.8 mM of calcium medium. Therefore, we postulated that IL-17 and IL-22 might play a role in proinflammatory responses in differentiated keratinocytes, via the induction of IKKα.
In order to characterize the relationship between IL-17 and IL-22 treatment and IKKα expression in vivo, we intradermally injected 30µg of IL-17 or IL-22 into the mice ears. After 48 hours, the tissues were collected and fixed for staining. Immunohistochemical staining revealed marked increases in IKKα in the mouse ears treated with IL-17 or IL-22, compared to those treated with PBS (Fig. 6). Additionally, the mouse ears that elevated IKKα levels evidenced inflammatory pathology of the skin, including thickened dermal layers with inflammatory cell infiltrations. These results are consistent with the observation that IL-17 and IL-22 promote skin inflammation; according to our results, IKKα also appears to contribute to the inflammatory responses in the skin, following the treatment with Th17 cytokines.
In this study, we determined that elevated calcium concentration induced IKKα expression and terminal differentiation with a cell cycle arrest in HaCaT cell cultures. Although the induction of IKKα was accompanied by keratinocyte differentiation via calcium stimuli, IL-17 and IL-22 did not detectably influence the calcium-mediated differentiation or the cell cycle. Rather, IL-17 and IL-22 contributed to the inflammation via the induction of IKKα, followed by the secretion of pro-inflammatory cytokines, such as IL-8 or TNF-α in keratinocytes or skin layers.
As reported previously, when HaCaT cells were transferred to low calcium concentrations (0.03 mM) and maintained for 3 weeks, the HaCaT cells reverted to basal state and lost cytokeratin 1 expression10. Therefore, we cultured and maintained HaCaT cells at a calcium concentration of 0.06 mM for more than 3 weeks, and assessed the expression of involucrin, and cytokeratins 5 and 10. As no differentiation markers were detected, we used the cells for the experiments.
IKKα induces keratinocyte differentiation by producing soluble factors, but operates independently of NF-κB. Therefore, the kinase activity of IKKα or interaction with IKKγ is not required for the induction of keratinocyte differentiation8. IKKα affected the secretion of keratinocyte differentiation inducible factor (kDIF), which induced terminal differentiation in IKKα-/- keratinocytes and mice8. Ultimately, IKKα and IKKα-mediated keratinocyte differentiation play a role in the immune surveillance by maintaining the skin homeostasis and preventing skin tumors via a variety of mechanisms, including the induction of kDIF, and by affecting the skin microenvironment via the regulation of inflammation13.
Interestingly, IL-17-treated or IL-22-treated HaCaT cells maintained at high concentrations of calcium evidenced increased IKKα expression. However, no significant differences in PI were noted among the cells in the control group, IL-17, or IL-22 after switching to a calcium concentration of 2.8 mM, and these differences persisted for 5 days. Although it has been reported previously that Th17 cytokines, particularly IL-17 and IL-22, mediate a distinct downstream pathway, which leads to a psoriatic phenotype; i.e., IL-17 is more proinflammatory; whereas, IL-22 retards keratinocyte differentiation3,5. Therefore, our findings appear to indicate that IL-17 or IL-22 affected differentiated keratinocytes by inducing proinflammatory responses via IKKα, and IL-17 and IL-22 stimuli did not seem to affect further differentiation of keratinocytes via calcium stimulus.
Th17 cells are known to be involved in the pathogenesis of certain human and animal chronic inflammatory skin diseases, including psoriasis14. Th17 cells are induced by exposure to TGF-β, IL-6, IL-15, and IL-2315. Th17 cells are a separate lineage of T cells, which produce IL-17A, IL-17F, TNF-α, IL-21, and IL-22. IL-17 and IL-22 induce the expression of β-defensin, S100A7 (psoriasin), S100A8, S100A9, as well as LL37 (cathelicidin) in keratinocytes2,16. In this study, we demonstrated that IL-17 and IL-22 induced IL-1β, IL-8 or TNF-α secretion via IKKα expression. This suggests that through the induction of IKKα, IL-17 and IL-22 affected the pathogenesis of inflammatory skin disease, e.g., psoriasis, by inducing inflammation via secretion of pro-inflammatory cytokines or possibly antimicrobial peptides and chemokines from the differentiated keratinocytes.
Figures and Tables
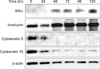 | Fig. 1IKKα expression is increased under a calcium concentration of 2.8 mM, according to the immunoblot results. HaCaT cells in 0.06 mM of calcium medium were induced to differentiation by replacement with high calcium medium (2.8 mM), and samples were collected every 24 hours for protein extraction. Involucrin, cytokeratin 5, and cytokeratin 10 were detected by the immunoblotting technique described in the Materials and Methods section. Beta-actin blotting was conducted as a loading control. |
 | Fig. 2Cell cycle analysis demonstrates that HaCaT cells cultured by switching into high calcium concentrations (Ca 2.8 mM) evidenced an elevated proportion of G0/G1 phase, regardless of IL-17 and IL-22 treatment. By way of contrast, HaCaT cells maintained at low calcium concentrations (Ca 0.06 mM) evidenced more cells in S- or G2/M phase compared with those transferred to a medium containing 2.8 mM of calcium. The data are expressed as the means±standard error of the mean and the statistical differences were analyzed between low- and high-calcium treated groups in each of the experiments. PI=(S+G2/M)/(G0/G1+S+G2/M). NS: not significant, PI: proliferation index. **p<0.01; *p<0.05. |
 | Fig. 3The effects of IL-17 and IL-22 treatment on the expression of IKKα and phosphorylated IκB in HaCaT cells. Cells were treated with IL-17 and IL-22 up to a concentration of 100 ng/ml in a calcium concentration of 2.8 mM and cultured for 24 hours. (A) The immunoblot shown is representative of three independent experiments. (B) The pixel densities are divided by β-actin densities to correct the values, and the data are expressed as the means±standard error of the mean at three independent experiments. *p<0.05. |
 | Fig. 4IL-17 and IL-22 induce pro-inflammatory cytokine production in HaCaT cells. HaCaT cells were cultured under 100 ng/ml of IL-17 or IL-22 for 48 hours, with or without preincubation of PS1145, at a concentration of 10µM for an hour and collected culture supernatant for ELISA. The data are expressed as the means±standard error of the mean at three independent experiments. NS: not significant. *p<0.05. |
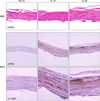 | Fig. 5Both IL-17 and IL-22 induce an increase in IKKα expression in Neoderm. Neoderm tissues were cultured under 100 ng/ml of IL-17 or IL-22 for 48 hours and fixed for H&E and immunohistochemical staining assays. The results shown are representative of the three independent batches of Neoderm experiments. |
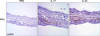 | Fig. 6Elevated IKKα levels in inflammatory mice ears, evoked by IL-17 and IL-22 injection. Skin inflammation, edema, and acanthosis, which were involved in the infiltration of neutrophils and dendritic cells into the ear skin of C57BL/6 WT mice, but less profound inflammatory changes, were noted in the sham controls. Each ear of C57BL/6 WT mice were injected intradermally with 3µg of IL-17 and IL-22 for two consecutive days in a total volume of 30µl. The control group of mice was injected with the same volumes of phosphate-buffered saline (PBS) via an identical route. |
ACKNOWLEDGEMENT
This work was supported by the Basic Research in Medicine from Korean Institute of Medicine and GlaxoSmithKline (Grant No. KG 2010-17).
References
1. Tonel G, Conrad C. Interplay between keratinocytes and immune cells--recent insights into psoriasis pathogenesis. Int J Biochem Cell Biol. 2009. 41:963–968.

2. Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, et al. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest. 2008. 118:597–607.

3. Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suárez-Fariñas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008. 159:1092–1102.

4. Zheng Y, Danilenko DM, Valdez P, Kasman I, Eastham-Anderson J, Wu J, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007. 445:648–651.

5. Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, Morel F. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J Immunol. 2005. 174:3695–3702.

6. Mauro T, Bench G, Sidderas-Haddad E, Feingold K, Elias P, Cullander C. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: quantitative measurement using PIXE. J Invest Dermatol. 1998. 111:1198–1201.

7. Elias PM, Ahn SK, Denda M, Brown BE, Crumrine D, Kimutai LK, et al. Modulations in epidermal calcium regulate the expression of differentiation-specific markers. J Invest Dermatol. 2002. 119:1128–1136.

8. Hu Y, Baud V, Oga T, Kim KI, Yoshida K, Karin M. IKKalpha controls formation of the epidermis independently of NF-kappaB. Nature. 2001. 410:710–714.

9. Sil AK, Maeda S, Sano Y, Roop DR, Karin M. IkappaB kinase-alpha acts in the epidermis to control skeletal and craniofacial morphogenesis. Nature. 2004. 428:660–664.

10. Deyrieux AF, Wilson VG. In vitro culture conditions to study keratinocyte differentiation using the HaCaT cell line. Cytotechnology. 2007. 54:77–83.

12. Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002. 277:16639–16647.
13. Liu B, Zhu F, Xia X, Park E, Hu Y. A tale of terminal differentiation: IKKalpha, the master keratinocyte regulator. Cell Cycle. 2009. 8:527–531.

14. Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, et al. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol. 2009. 182:1631–1640.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download