Abstract
Alopecia areata (AA) is an inflammatory hair loss of unknown etiology. AA is chronic and relapsing, and no effective cure or preventive treatment has been established. Vitamin D was recently reported to be important in cutaneous immune modulation as well as calcium regulation and bone metabolism. It is well known that areata is common clinical finding in patients with vitamin D deficiency, vitamin D-resistant rickets, or vitamin D receptor (VDR) mutation. The biological actions of vitamin D3 derivatives include regulation of epidermal cell proliferation and differentiation and modulation of cytokine production. These effects might explain the efficacy of vitamin D3 derivatives for treating AA. In this study, we report a 7-year-old boy with reduced VDR expression in AA, recovery of whom was observed by topical application of calcipotriol, a strong vitamin D analog.
Hair follicles are highly sensitive to hormones1. Vitamin D is a hormone that plays an important role in calcium homeostasis, immune regulation, and cell growth and differentiation1. The active form of vitamin D, 1,25-dihydroxyvitamin D3, mediates its action by binding to specific vitamin D receptors (VDR) located in the nuclei of target cells2. VDR is a member of the nuclear hormone receptor superfamily that acts as a ligand-inducible transcription factor regulating vitamin D-responsive genes1. It has been demonstrated that VDR is strongly expressed in the key structures of human and murine hair follicles1. A lack of VDR is associated with reduced epidermal differentiation and hair follicle growth3. Expression of VDR in keratinocytes is necessary for maintenance of the normal hair cycle3. In addition, patients with hereditary 1,25-dihydroxyvitamin D3-resistant rickets type II and VDR knockout mice exhibit a phenotype that includes alopecia4,5.
Alopecia areata (AA) is an inflammatory hair loss of unknown etiology. A role of the immune system in the pathogenesis of this disease is supported by strong evidence. Although several treatment modalities appear to be effective, some severe cases do not achieve complete response and may relapse after therapy. The duration of complete response to therapy may be related to a patient's inherent immune response rather to the treatment used. A therapeutic effect of vitamin D analog on hair loss in neonatal rats was recently reported3. However, the expression pattern of VDR in AA of the human scalp has not yet been reported. In this case, we found reduced VDR expression in AA, recovery from which was observed after topical application of calcipotriol, a strong vitamin D analog.
A 7-year-old otherwise healthy boy presented with a 2-month history of sudden hair loss on the vertex region of the scalp (Fig. 1a). On physical examination, a single well-demarcated patch of alopecia was present in the vertex area. Yellow dots and exclamation hairs were observed at folliscopy and dermoscopy. Trichotillomania should be kept in mind when AA is considered; however, we confirmed the latter diagnosis by histopathological examination. Topical minoxidil 5% and 1% hydrocortisone cream were applied for 3 months to treat the AA, but the lesions did not respond well to treatment.
After obtaining written informed consent from the patient's parents, we prescribed calcipotriol solution (Daivonex, 50µg/ml) to be applied once daily for 3 months. Before the treatment, a 4 mm punch biopsy was performed of the bald patch on the scalp and immunohistochemical staining for VDR was performed, but VDR expression was not observed in the hair follicles (Fig. 2a). Initial new hair growth was found at 6 weeks after initial application of calcipotriol. After 3 months of calcipotriol therapy, complete regrowth was observed in the affected area (Fig. 1c). Punch biopsies (4 mm) were re-obtained from the affected scalp lesions. Immunohistochemical staining for VDR was performed, and VDR was detected in some nuclei of the keratinocytes in the hair follicles (Fig. 2b). No hair loss relapse was observed over the next 6 months.
AA is difficult to treat. According to the guidelines of the British Association of Dermatologists, contact immunotherapy and corticosteroids are the most effective and best documented, but even these treatments often fail to induce hair growth6. Therefore, the need exists for more efficient treatments.
The mechanism of topical calcipotriol in inducing hair regrowth in AA lesions is thought to regulate the differentiation of B cells, T cells, dendritic cells, and the expression of Toll-like receptors7. Growing evidence suggests that use of vitamin D may be helpful in several autoimmune diseases like multiple sclerosis, type 1 diabetes mellitus, lupus, and rheumatoid arthritis8.
Vitamin D has a multitude of biological effects that interact with the innate and adaptive immune system, most of which lead to its downregulation7. Vitamin D has direct effects on T and B cells and shapes their responses to activation. The effect of 1, 25-dihydroxycholecalciferol [1, 25(OH)2D3] on the acquired antigen-specific immune response is T lymphocyte proliferation inhibition, particularly of the Th1 arm9. The addition of 1, 25(OH)2D3 to CD4 T cells inhibits Th1 cell proliferation and cytokine production10 and leads to decreased secretion of interleukin (IL)-2 and interferon-γ by CD4 T cells and promotes IL-5 and IL-10 production, which further tilts the T cell response toward Th2 dominance11. Vitamin D has also been shown to inhibit antibody secretion and autoantibody production in B cells12.
Dendritic cells play a central role in regulating immune activation and responses to self9. Dendritic cell maturation is central to the outcome of antigen presentation to T cells9. In vitro, 1, 25(OH)2D3 inhibits the differentiation of monocytes into dendritic cells and impedes T cell-induced stimulatory activity13. It has been shown that 1, 25(OH)2D3 is one of the most powerful blockers of dendritic cell differentiation and of IL-12 secretion. IL-12 inhibition is achieved through the direct interaction of 1, 25(OH)2D3 bound to the VDR, which interferes with nuclear factor-kappaB-induced transcription of IL-1214. Therefore, vitamin D is not the only factor affected by exposure to sunlight that has the capacity to modify immune function. A clinical trial of topical vitamin D analogs for the treatment of AA might be very helpful.
Another possible mechanism is that topical application of vitamin D might play a role in restoration of hair cycle dysfunction in AA. Recently, Xie et al.3 reported that VDR is required for the critical stage of secondary hair follicle development. The development of alopecia in the case of VDR deficiency but not vitamin D deficiency remains unexplained; however, it has been suggested that VDR has ligand-independent roles that are critical to hair follicle cycling3. The biological actions of vitamin D3 derivatives include regulation of epidermal cell proliferation and differentiation and modulation of cytokine production15. These effects might explain the efficacy of vitamin D3 derivatives for treating AA.
Our patient with AA did not respond to various treatments, including topical and intralesional corticosteroids. We tried calcipotriol as another treatment option to stimulate impaired function of VDR in AA. VDR is not expressed in only epidermal keratinocytes, but it is also found in the outer root sheath keratinocytes and dermal papilla cells of hair follicles1. Furthermore, the enzyme for synthesizing 1, 25(OH)2D3, 25-hydroxyvitamin D-1a-hydroxylase, is expressed in both the basal layer of the epidermis and the matrix cells of hair follicles in the dermis, suggesting that keratinocytes in hair follicles both make and respond to their own 1,25(OH)2D316.
In this case, we have shown that AA presents with reduced VDR expression and that topical application of a vitamin D analog might be another treatment option upon failure of conventional therapies. To our knowledge, this is the first report of the successful treatment of AA with topical application of a vitamin D analog. Further studies are needed to assess the effectiveness of this therapeutic modality in a greater number of patients with AA.
Figures and Tables
 | Fig. 1(a) Central view of a 7-year-old patient with alopecia areata (AA) before treatment. (b) The same patient after 1 month of treatment with topical calcipotriol. (c) Calcipotriol ointment treatment resulted in complete clinical remission after 3 months of treatment in a case of AA. |
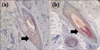 | Fig. 2(a) Punch biopsies (4 mm each) were obtained from the bald patch on the scalp before the start of treatment. Immunohistochemical staining for vitamin D receptor (VDR) was performed, but black arrow VDR expression was not observed in the hair follicles (immunohistochemical staining for VDR, ×200). (b) After 3 months of calcipotriol therapy, 4-mm punch biopsies were re-obtained from the affected scalp lesion. Immunohistochemical staining for VDR was performed, and black arrow positivity was detected in some nuclei of the keratinocytes in the hair follicles (immunohistochemical staining for VDR, ×200). |
ACKNOWLEDGMENT
This study was supported by a Korea Research Foundation Grant funded by the Korean Government (2010-0021960).
References
1. Reichrath J, Schilli M, Kerber A, Bahmer FA, Czarnetzki BM, Paus R. Hair follicle expression of 1,25-dihydroxyvitamin D3 receptors during the murine hair cycle. Br J Dermatol. 1994. 131:477–482.

2. Reichel H, Koeffler HP, Norman AW. The role of the vitamin D endocrine system in health and disease. N Engl J Med. 1989. 320:980–991.

3. Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002. 118:11–16.

4. Sakai Y, Kishimoto J, Demay MB. Metabolic and cellular analysis of alopecia in vitamin D receptor knockout mice. J Clin Invest. 2001. 107:961–966.

5. Forghani N, Lum C, Krishnan S, Wang J, Wilson DM, Blackett PR, et al. Two new unrelated cases of hereditary 1,25-dihydroxyvitamin D-resistant rickets with alopecia resulting from the same novel nonsense mutation in the vitamin D receptor gene. J Pediatr Endocrinol Metab. 2010. 23:843–850.

6. MacDonald Hull SP, Wood ML, Hutchinson PE, Sladden M, Messenger AG. British Association of Dermatologists. Guidelines for the management of alopecia areata. Br J Dermatol. 2003. 149:692–699.

7. Nancy AL, Yehuda S. Prediction and prevention of autoimmune skin disorders. Arch Dermatol Res. 2009. 301:57–64.

8. Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part II. Treatment. J Am Acad Dermatol. 2010. 62:191–202.
9. Arnson Y, Amital H, Shoenfeld Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Ann Rheum Dis. 2007. 66:1137–1142.

10. Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O'Garra A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001. 167:4974–4980.

11. van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005. 97:93–101.

12. Linker-Israeli M, Elstner E, Klinenberg JR, Wallace DJ, Koeffler HP. Vitamin D(3) and its synthetic analogs inhibit the spontaneous in vitro immunoglobulin production by SLE-derived PBMC. Clin Immunol. 2001. 99:82–93.

13. Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001. 98:6800–6805.

14. D'Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998. 101:252–262.
15. Segaert S, Simonart T. The epidermal vitamin D system and innate immunity: some more light shed on this unique photoendocrine system? Dermatology. 2008. 217:7–11.

16. Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001. 86:888–894.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download