Abstract
Background
There are several commercially available agents to treat female pattern hair loss (FPHL), including minoxidil solution, anti-androgen agents and mineral supplements. However, these treatments are not always satisfactory. We report the results of a clinical trial of 17α-estradiol (Ell-Cranell® alpha 0.025%) solution to Korean female patients with FPHL.
Objective
This study was designed to examine the efficacy and safety of Ell-Cranell® alpha 0.025% solution in Korean female patients with FPHL.
Methods
A total of 53 women, 18 to 55 years old, applied topical Ell-Cranell® alpha 0.025% solution once daily for 8 months. Efficacy was evaluated by the change of hair counts and diameter, subjective assessment, and photographic assessment by investigators.
Results
Hair counts and diameter from baseline to 4 and 8 months after treatment increased in treated patients and these changes were statistically significant (p<0.0001). 17α-estradiol (Ell-Cranell® alpha 0.025%) solution showed significant improvement by subjective self-assessment and by investigator photographic assessment. Ell-Cranell® alpha 0.025% solution was well tolerated over 8-months period.
Androgenetic alopecia is the thinning of hairs and the reduction of hair density related to male hormones (androgens) in both males and females who are genetically predisposed to the condition. It is the most common cause of alopecia in males as well as in females1. Androgenetic alopecia has been shown to be a hereditary hypersensitivity to the male hormone, testosterone. While the causes in males and females are similar, the display patterns are different2. In males, the hair line at the temple becomes vague, hairs at the vertex are decreased and are thinly connected, resulting in increasingly obvious bald areas. On the other hand, in females, while the frontal hairline is maintained, hair density in the frontal area and the temporal region is decreased2.
Androgenetic alopecia has been shown to be more common in males. According to one Korean survey, 5.6% of 4,601 females showed androgenetic alopecia that was higher than grade I according to the Ludwig classification, an ample number of female pattern hair loss patients3. The severity of hair loss in those with female pattern hair loss is milder than that of male pattern hair loss. Nevertheless, it has a major effect on women, who are generally more conscious about their appearance, potentially impairing their social lives and resulting in psychological pain. Currently, to treat such female pattern hair loss, anti-androgen agents, topical minoxidil agents and mineral supplements have been used. However, depending on the patient, treatment outcomes are not always satisfactory4.
The solution 0.025% Ell-Cranell® alpha (17α-estradiol, Galderma Korea, Co., Seoul, Korea) is a stereoisomer of the female hormone 17β-estradiol and has been used for the past 30 years in Europe, as well as in South America. The drug inhibits the conversion of testosterone to the metabolite dihydrotestosterone (DHT) by suppressing 5α-reductase activity5. In addition, by inhibiting 17β-dehydrogenase, it impedes the conversion process of androstenedione to testosterone, resulting in a reduction in the syntheses of testosterone and DHT6. It also accelerates the conversion of testosterone to estradiol by stimulating aromatase, decreasing the level of testosterone and leading to a reduction in DHT7. In addition, the drug has been reported to stimulate the generation of hair follicular matrix cells8. Clearly the use of 0.025% Ell-Cranell® alphasolution on decreased hair loss in patients with androgenetic alopecia has been shown both effective and safe. Nonetheless, the drug is not imported into Korea, and studies on Korean patients have not been conducted. Therefore, we conducted this study to assess the safety and effectiveness of 0.025% Ell-Cranell® alphasolution in Korean patients with female pattern hair loss.
This study was an open-labeled, single-arm, single institution clinical trial. It was performed from March 2010 to December 2010 after obtaining approval from the Institutional Review Board of Wonju Christian Hospital, Wonju College of Medicine, Yonsei University.
The subjects were female androgenic hair loss patients between the ages of 18 and 55 years, who visited the Department of Dermatology at Wonju Christian Hospital and diagnosed as lower specific type F1 or F2 according to the basic and specific (BASP) classification9. Patients with dermatological or systemic diseases which could have affected the results of the trial were excluded. Patients who had not used hair restorers for the treatment of hair loss for a minimum of six months prior to the initiation of the trial and patients who were not taking any medication that would influence the results, as determined by the investigators, were recruited. The clinical trial was explained in detail to patients who participated in the study, participation was decided by the patients themselves and written consent was obtained.
In our study, 0.025% Ell-Cranell® alphasolution was used to treat hair loss. Considering that this is a drug for which the effectiveness for hair restoration has been demonstrated, is currently in use and is in phase 4 of the drug review process, as well as being approved by the Korean Food and Drug Administration, a control group for the application of a placebo was not included10. The experimental drug was applied once a day at 3 ml/application using a pre-dosed applicator, and the head was massaged for approximately one minute to facilitate the absorption of the drug. The experimental drug was a topical agent, and the subjects were instructed to apply the experimental drug only to the scalp.
The subjects used 0.025% Ell-Cranell® alphasolution for eight months. The first efficacy evaluation was performed by examining the change in the number of hairs as assessed by phototrichogram (Folliscope®, Lead M Co., Seoul, Korea). For the second efficacy evaluation, performed four months after the initial application, the change in the number of hairs and the diameter of hair was again evaluated using phototrichogram. The growth of hair was also evaluated by the subjects themselves by questionnaire. In addition, the growth and loss of hairs were evaluated by Global photography. After using the trial drug for eight months, the change in the diameter of hairs was again evaluated.
Prior to photographic documentation, the hairs were combed in order to expose all areas of hair loss. Clinical photographs were taken using a phototrichogram with constant film emulsion, contrast, frame, exposure and reproduction rate, while the head of subjects were immobilized.
The paired photographs were evaluated by investigators. For example, the baseline photos were compared and analyzedversus photos taken at two, four, six or eight months. The photographs were evaluated on a scale of 'greatly improved' to 'worsened.' The scale was defined as follows: 'greatly improved' was an improvement of more than 75%, 'moderately improved' was 50~75% growth, 'slightly improved' was 25~50%, 'no change' meant less than a 25% improvement and 'worsened' meant deteriorated cases.
To examine the change in the hairs from the first visit to four and eight months after application, the thickness and density of hairs before and after the application were compared by phototrichogram, and treatment effects were analyzed objectively. At the first visit, the area with the most severe hair loss was tattooed with black dots, and photographs were taken with these dots positioned in the center of the phototrichogram. The number of total hairs in a unit area (hair density) was measured by calculating the number of all hairs within the 70 mm2 circle. The diameter of the thickest five hairs was measured using a 200× lens and was presented as the average thickness of hair.
Two types of questionnaire were administered at two, four, six and eight months to determine whether the medication was producing esthetically acceptable results, how the subjects viewed the effects and their satisfaction levels. The level of improvement in hair loss and the level of satisfaction were evaluated using a visual analogue scale, and the results regarding the level of improvement and the satisfaction level were obtained in such a way so as to be analyzed and presented as percentages.
Twelve and 24 weeks after the application, the presence or absence of side effects was assessed by way of subject interviews. In cases where side effects were reported, the level of severity and the cause-and-effect relationship with the study drug were examined. In addition, any discomfort or side effects felt by the subjects were examined with open questions within the questionnaire.
The investigators evaluated topical safety at baseline and then again at two, four, six and eight months. Included in topical tolerance category were erythema, pruritus, tingling sensation and desquamation. The investigators evaluated these conditions directly (erythema, desquamation) or by interviewing the subjects (pruritus, tingling sensation) and scored each variable.
We evaluated all events related to the safety of patients who had applied the experimental drug more than once. All abnormal reactions that developed after the application of the drug and the frequency of the reactions were evaluated. We then assessed all abnormal drug reactions, severe abnormal reactions, unexpected abnormal drug reactions and the number of patients who dropped out due to abnormal reactions.
Unless stated otherwise, the significance level for statistical analysis was 0.05, and two-tail tests were performed.
The subject group included 51 patients who applied the experimental drug more than once and participated for the full duration of the study, allowing an efficacy evaluation to be performed.
Study participants registered prior to participating in the trial. Age and other demographic factors were assessed.
At the first efficacy evaluation, all subjects used 0.025% Ell-Cranell® alphasolution for eight months, and the change in the number of hairs assessed by phototrichogram was analyzed by paired t-test or Wilcoxon signed rank test. For the analysis of the second safety evaluation variables at four months, the change in the number of hairs and the diameter of hairs were assessed by phototrichogram and analyzed by paired t-test or Wilcoxon signed rank test. In addition, descriptive statistics were generated based on the evaluation of hair growth as described by the subjects at two months (questionnaire Type I and II), four months (questionnaire Type II), six months (questionnaire Type II), and eight months (questionnaire Type I and II). In addition, at two, four, six and eight months, descriptive statistical analysis was performed on the overall self-evaluation of hair growth and loss by paired t-test or Wilcoxon signed rank test (Table 1).
A total of 53 subjects were recruited. One patient dropped out just prior to initiating the study, and another patient cancelled prior to the first efficacy evaluation, both for personal reasons. Thus, 51 patients participated in the study. Therefore, the study included 51 patients who were repeatedly treated with the experimental drug and with whom the efficacy evaluation could be performed.
The average age of the subjects who participated in the study was 41.61 (±8.60) years. More than 50% of the subjects were in their 40s. The proportion of subjects with a family history of androgenetic alopecia was 62.74% (32/51 patients). The proportion of subjects who met the criteria for F1 of the androgenetic alopecia classification by the BASP classification was 66.66% (34/51 patients), the remaining patients were classified as F2. Patients who met the criteria for F3 were excluded from the selection criteria (Table 2).
A solution of 0.025% Ell-Cranell® alphawas applied to androgenetic alopecia subjects for eight months prior to the first efficacy evaluation. The change in the number of hairs was assessed by phototrichogram, and the following results were obtained (Fig. 1). The average number of baseline hairs was 323.59 (±55.52) hairs/cm2, and the average number of hairs after eight months of drug application was 355.16 (±61.40) hairs/cm2. In comparison with the baseline, the number of hairs after eight months of drug application was increased by an average of 31.57 (±34.63) hairs/cm2, a statistically significant (p<0.0001) change.
The number of hairs after four months of drug application was 341.39 (±56.66) hairs/cm2. In comparison with the baseline, the number of hairs at four months increased by an average of 17.80 (±22.96) hairs/cm2, and the change was statistically significant (p<0.0001) (Fig. 1).
The results of the analysis of the change in hair diameter as assessed by phototrichogram after four and eight months of treatment are shown in Fig. 2. The average diameter of hair at the baseline was 78.45 (±10.62) µm, the average diameter of hair after four months of drug application was 84.84 (±9.70) µm, and the average diameter of hair after eight months of drug application was 88.84 (±10.48) µm. In comparison to baseline, after four months of using the study drug, the diameter of the hairs, as assessed by phototrichogram, increased by an average of 6.39 (±8.21) µm. After eight months, the diameter of the hair that was assessed by phototrichogram increased by an average of 10.39 (±10.08) µm, and the changes were statistically significant (p<0.0001).
The type I evaluation was conducted by subjects after two and eight months of drug use. Table 3 shows the categories in which more than 60% of the subjects answered 'strongly agree' or 'agree' and the associated rates in comparison to prior to drug application. The percentage of subjects who agreed at two months to the statement 'the product could be applied easily' was 82.35% (42/51 patients), and 84% (42/50 patients) gave this response at eight months. The percentage of subjects at two months who agreed to the statement 'the time needed for the skin to absorb the product was satisfactory' was 82.31% (43/51 patients), and 84% (42/50 patients) reported such at eight months. The percentage of subjects at two months who agreed to the statement 'the use of the product does not render the hair or scalp oily' was 64.70% (33/51 points), and 76% (38/50 patients) stated this at eight months. The percentage of subjects at two months who agreed to the statement 'the product does not render the scalp or hair sticky' was 76.47% (39/51 patients), and 82% (41/50 patients) answered in this way at eight months. The percentage of subjects who agreed at two months to the statement 'the product does not induce pruritus in the scalp' was 78.43% (40/51 patients), and 80% (40/50 patients) stated such at eight months. The percentage of subjects at two months who answered 'agree' to the statement 'the product does not induce a tingling sensation or scalp irritation' was 76.47% (39/51 patients), and 70% (35/50 patients) responded thus at eight months. The percentage of subjects at two months who answered 'agree' to the statement 'the product does not leave residue on the scalp' was 82.35% (42/51 patients), and that at eight months was 78% (39/50 patients). The percentage of subjects at two months who answered 'agree' to the statement 'overall, the product is good to use' was 82.35% (42/51 patients), and 80% (40/50 patients) gave this response at eight months.
On the other hand, for the category of product smell, 'very good' or 'good' was reported at two months for 15.68% (8/51 patients) of patients and at eight months by 24% (12/50 patients). At two months, as well as eight months, most subjects responded 'disagree' to the above statement. In addition, in response to the statement 'after product application, my hair is difficult to comb,' 5.88% of subjects responded 'strongly agree' or 'agree' at two months (3/51 patients), and 14% (7/50 patients) replied in this manner at eight months. At both two and eight months, the highest percentage of subjects responded 'disagree' to this statement.
The results of the evaluation (Type II) performed by the subjects at two, four, six and eight months after the application of drug are shown in Table 4. In comparison with baseline, at eight months after the application of drug, the percentage of the subjects who responded 'yes' to the statement 'after using the product, hair loss was reduced' was 54% (27/50 patients); those who responded 'no' was 24% (12/50 patients). At eight months, for the question 'after using the product, did hair grow again?', the percentage of the subjects who responded 'yes' was 32% (16/50 patients), while those who answered 'no' was 38% (19/50 patients). Again at eight months, regarding the question 'after using the product, was the progression of hair loss slowed?', the percentage of the subjects who answered 'yes' was 58% (29/50 patients), and subjects who responded 'no' was 18% (9/50 patients). For the questions 'in comparison with baseline, is the eight month hair density in the frontal area satisfactory?,' the percentage of the subjects who responded 'yes' was 22% (11/50 patients), and that of the subjects who responded 'no' was 44% (22/50 patients). At eight months, in response to the question 'in comparison with baseline, is the hair density in the vertex area satisfactory?', the percentage of subjects who responded 'yes' was 18% (9/50 patients), while 58% answered 'no' (29/50 patients). At eight months, regarding the question 'in comparison with baseline, is the overall hair shape (appearance) satisfactory?,' the percentage of the subjects who responded 'yes' was 24% (12/50 patients), and that of the subjects who responded 'no' was 42% (21/50 patients). In the question 'after using the product, how is the appearance of your hair?,' the percentage of subjects at eight months who evaluated their appearance as 'improved' was 34% (17/50 patients), and none of subjects responded 'worsened'.
The satisfaction level with hair loss did not show a constant trend at any particular time point. However, overall, the hair loss and hair restoration effects were best at eight months, and the satisfaction levels of subjects at each time point were similar. In addition, the morphological change in hair loss and hair restoration effects were improved in more than 30% of the subjects. Nonetheless, the satisfaction level of subjects with reduced hair loss was relatively low.
The results of the overall evaluation performed by the investigators at two, four, six and eight months after drug application are shown in Fig. 3 and 4. In comparison with baseline, two months after using the study drug, the percentage of subjects who evaluated themselves as 'greatly/moderately/slightly improved' was 1.96% (1/51 patients); at four months, this increased to 35.29% (18/51 patients); at six months, the proportion further increased to 56% (28/50 patients); and that at eight months was 80% (40/50 patients). In addition, at all time points, none of subjects responded that their condition had 'worsened.' In comparison with the categories 'no change' or 'worsened,' the percentage of subjects who estimated 'greatly/moderately/slightly improved' increased gradually with time.
For the safety evaluation, safety, abnormal reactions and incidence of abnormal reactions during topical use were calculated for all subjects treated numerous times with the experimental drug. The proportion of subjects who developed abnormal reactions after applying the drug was 34.61% (18/52 patients, 36 cases). Abnormal drug reactions related to the experimental drug were reported in 3.84% of subjects (2/52 patients, five cases). None of patients dropped out due to the abnormal reactions. The abnormal drug reactions which had a causal relationship with the experimental drug were an itching sensation and alopecia observed in one subject. Another subject reported irritation and erythema at the application site, determined to be directly related to the study drug. In the assessment of the topical reactions that are known side effects of 0.025% Ell-Cranell® alpha solution (erythema, tingling sensation, pruritus and desquamation), none of subjects experienced pruritus or desquamation at any time point after application of the drug. At two months, one subject developed mild erythema and a tingling sensation. After four, six and eight months of applying the study drug, none of these symptoms were reported.
In conclusion, applying 0.025% Ell-Cranell® alpha solution to female pattern hair loss subjects did not produce any unsafe reactions that required special attention or any unexpected drug side effects. In addition, all of the reactions that were evaluated as abnormal drug reactions were local reactions in the application area, and no systemic reactions were observed.
Androgenetic alopecia which occurs in females is referred to as female pattern hair loss. The cause of androgenetic alopecia is hereditary hypersensitivity of the hair roots to the male hormone, testosterone. The severity of female pattern hair loss is milder than that of male pattern hair loss. Nonetheless, it has a tremendous effect on appearance, interferes with the social lives of patients, and may result in psychological pain. In addition, due to the aging of the population in general and the increasing number of women having a social life at an older age, the demand for a treatment for female pattern hair loss is rapidly increasing2-4. Various therapeutics have been tried for the treatment of female pattern hair loss; however, treatment outcomes are not always satisfactory, and further studies for the development of new therapeutics are needed4.
Until now, a variety of factors have been suggested as the cause of female pattern hair loss. It has been reported that thyroid diseases or a reduction in serum ferritin are associated with female pattern hair loss. It is thought that androgen plays the major role in female pattern hair loss11-13. Several studies have reported that, in the hair follicles in the alopecia areas of androgenetic alopecia patients, 5α-reductase activity, DHT concentration and androgen receptors are elevated in comparison with the levels in normal individuals14-18. Testosterone is converted to DHT by 5α-reductase, and due to the action of DHT, a reduction in the sizes of hair follicles and a shortening of the growth period occurs, causing further hair loss19. In females, as compared to males, the expression of the 5α-reductase androgen receptor in the hair follicles in the frontal region is low, and P-450 aromatase, which converts testosterone to estradiol, is abundant. Thus, relative mild hair loss results in. However, it has been also reported that, even in female pattern hair loss patients, the concentrations of testosterone and serum dehydroepiandrosterone sulfate are higher than those in a normal control group20,21.
The clinical trial drug used in our study, 0.025% Ell-Cranell® alphasolution, is a stereoisomer of the female hormone 17β-estradiol. Various experiments have shown that, in comparison with 17β-estradiol, 17α-estradiol did not exhibit estrogen activity or exhibited only very weak activity. In the treatment of androgenetic alopecia, the action mechanism of 17α-estradiol is to suppress 5α-reductase activity, which impedes the conversion of testosterone to the more potent metabolite DHT5,22,23. In addition, it inhibits 17β-dehydrogenase activity, resulting in a slowing of the conversion process of androstenedione to testosterone. As a result, there is a reduction in the syntheses of testosterone and DHT6. On the other hand, by stimulating aromatase, the conversion of testosterone to estradiol is accelerated, hence, testosterone is reduced. It thus acts to ultimately reduce DHT7. In addition, it has been reported to accelerate the generation of hair follicular matrix cells8.
The 0.025% Ell-Cranell® alpha solution has been used for the past 30 years in Europe and in South America. There were several reports about the therapeutic effects of 17α-estradiol in European population. In a controlled, randomized double-blind study, 63% of the treated patients with 17α-estradiol showed a reduction of the amount of telogen hairs, whereas in the control group the same reduction was found in only 37% of the cases23. Wozel et al.22 reported that 17α-estradiol increases and maintains the rate of anagen hair in 88% of the treated patients. When compared with minoxidil 17α-estradiol showed relatively lower efficacy compared to minoxidil in hair density and thickness5.
In our clinical trial, the subjects were female pattern hair loss patients between the ages of 18 and 55 years old who had been diagnosed with specific type F1 or F2 by the BASP classification, and the efficacy and safety of 0.025% Ell-Cranell® alphasolution were assessed9. The results of our clinical trial, during which 0.025% Ell-Cranell® alphasolution was applied to female pattern hair loss patients for eight months, showed that, after four and eight months of applying the study drug, the increases in the number of hairs and the diameter of hair, as assessed by phototrichogram, were statistically significant. Specifically, at eight months after the drug application, the number of hairs increased by 31.57 (±34.63) hairs/cm2 (95% confidence level: 21.83, 41.31) in comparison with baseline.
Our clinical study was a single-arm study, and thus interpretation of the results may be somewhat limited in comparison with studies that compared results to a placebo control group. Nonetheless, the results are comparable to the increased number of hairs (26.7 hairs/cm2) that was obtained after 32 weeks of applying 2% minoxidil in a double-blind, random assignment clinical study that was conducted previously in female androgenetic alopecia patients24.
At two and eight months after starting the study drug, the responses to the statements 'the product smells good' and 'after using the product, my hair is difficult to comb', more patients responded 'no' compared to those with affirmative responses. In other categories ('it is easy to apply the product,' 'the time for skin absorption of the product is satisfactory,' 'the use of the product did not render hair or scalp oily,' 'the product did not render the scalp or hair sticky,' 'the product did not induce pruritus in the scalp,' 'the product does not induce a tingling sensation or scalp irritation,' 'the product did not leave residue on the scalp' and 'overall, the product could be applied easily') positive responses represented more than 60% of the total.
The results for the evaluation of hair loss and hair restoration effects at two, four, six and eight months after the start of drug application did not show consistent trends. Overall, hair loss and hair restoration effects were shown to be best at eight months, and the satisfaction levels of patients were shown to be similar at each time point. The change in perceived appearance due to hair loss and hair restoration improved more than 30%. However, the satisfaction level of patients was relatively low. This could be because while hair loss was relatively improved, it was not completely cured. As the treatment period increased in duration, the expectation level for improvement increased.
The results of the analysis of the overall evaluation performed by the investigators at two, four, six and eight months after the start of drug use show that, in comparison with 'no change' or 'worsened,' the percentage of the subjects who evaluated themselves as 'greatly/moderately/slightly improved' gradually increased, showing that improvements in the alopecia condition were observed with time. In addition, the results of the evaluation at four and eight months showed an increase in the number of hairs and the diameter of hair when assessed by phototrichogram.
The results of the safety evaluation showed that abnormal drug reactions associated with the experimental drug were detected in 3.84% of the subjects (2/52 patients, 5 cases). All were mild local reactions, and there were no dropouts due to serious abnormal reactions or abnormal reactions. In the evaluation of the previously reported abnormal reactions caused by 0.025% Ell-Cranell® alpha solution (erythema, tingling sensation, pruritus, desquamation) that was performed at two, four, six and eight months, none of the patients experienced pruritus or desquamation. After two months of drug application, mild erythema and a tingling sensation were detected in one subject each (1.92%). However, the reactions were not reported again after four, six or eight months of drug application. Therefore, in the androgenetic alopecia patients who applied 0.025% Ell-Cranell® alpha solution for eight months, no noticeable abnormal reactions or events that violate safety regulations were observed. In addition, the observed abnormal drug reactions were all topical reactions in the application area, and no systemic reactions were observed. In conclusion, in this clinical study, the efficacy and safety of 0.025% Ell-Cranell® alpha solution were thoroughly evaluated, and this drug is considered to be a safe alternative for the effective treatment of female pattern hair loss.
Figures and Tables
 | Fig. 1Hair counts from baseline to 4, 8 months after treatment were increased in treated patients, statistically significant (p<0.0001). SD: standard deviation, Min: minimum, max: maximum, CI: confidence internal. |
 | Fig. 2Hair diameter from baseline to 4, 8 months after treatment were increased in treated patients, statistically significant (p<0.0001). SD: standard deviation, Min: minimum, max: maximum, CI: confidence interval. |
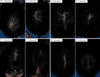 | Fig. 3Effectiveness of hair regrowth in patients with female pattern hair loss after 8 months treatment. Pt: patient. |
 | Fig. 4Investigator assessment of clinical response by duration of the treatment. 17α-estradiol (Ell-Cranell® alpha 0.025%) solution showed significant improvement by investigator photographic assessment. |
References
1. Sinclair R. Male pattern androgenetic alopecia. BMJ. 1998. 317:865–869.
2. Venning VA, Dawber RP. Patterned androgenic alopecia in women. J Am Acad Dermatol. 1988. 18:1073–1077.

3. Paik JH, Yoon JB, Sim WY, Kim BS, Kim NI. The prevalence and types of androgenetic alopecia in Korean men and women. Br J Dermatol. 2001. 145:95–99.

4. Shin HS, Lee SH, Kim DH, An JS, Kwon OS, Eun HC, et al. The efficacy and safety of AP-FHG0604T on female pattern hair loss: a randomized double-blind placebo-controlled clinical trial. Korean J Dermatol. 2007. 45:119–126.
5. Blume-Peytavi U, Kunte C, Krisp A, Garcia Bartels N, Ellwanger U, Hoffmann R. Comparison of the efficacy and safety of topical minoxidil and topical alfatradiol in the treatment of androgenetic alopecia in women. J Dtsch Dermatol Ges. 2007. 5:391–395.

6. Münster U, Hammer S, Blume-Peytavi U, Schäfer-Korting M. Testosterone metabolism in human skin cells in vitro and its interaction with estradiol and dutasteride. Skin Pharmacol Appl Skin Physiol. 2003. 16:356–366.

7. Hoffmann R, Niiyama S, Huth A, Kissling S, Happle R. 17alpha-estradiol induces aromatase activity in intact human anagen hair follicles ex vivo. Exp Dermatol. 2002. 11:376–380.

8. Arai A, von Hintzenstern J, Kiesewetter F, Schell H, Hornstein OP. In vitro effects of testosterone, dihydrotestosterone and estradiol on cell growth of human hair bulb papilla cells and hair root sheath fibroblasts. Acta Derm Venereol. 1990. 70:338–341.
9. Lee WS, Ro BI, Hong SP, Bak H, Sim WY, Kim do W, et al. A new classification of pattern hair loss that is universal for men and women: basic and specific (BASP) classification. J Am Acad Dermatol. 2007. 57:37–46.

10. Lew BL, Sim WY, Huh CH, Park JK. Clinical trial to evaluate the safety and efficacy of scalp med(R) in treatment of patients with androgenetic alopecia. Korean J Dermatol. 2008. 46:776–783.
11. Kantor J, Kessler LJ, Brooks DG, Cotsarelis G. Decreased serum ferritin is associated with alopecia in women. J Invest Dermatol. 2003. 121:985–988.

13. Sinclair R, Wewerinke M, Jolley D. Treatment of female pattern hair loss with oral antiandrogens. Br J Dermatol. 2005. 152:466–473.

14. Hoffmann R. Steroidogenic isoenzymes in human hair and their potential role in androgenetic alopecia. Dermatology. 2003. 206:85–95.

15. Hoffmann R. Enzymology of the hair follicle. Eur J Dermatol. 2001. 11:296–300.
16. Bang HJ, Yang YJ, Lho DS, Lee WY, Sim WY, Chung BC. Comparative studies on level of androgens in hair and plasma with premature male-pattern baldness. J Dermatol Sci. 2004. 34:11–16.

17. Zouboulis CC, Degitz K. Androgen action on human skin - from basic research to clinical significance. Exp Dermatol. 2004. 13:Suppl 4. 5–10.

18. Meisheri KD, Cipkus LA, Taylor CJ. Mechanism of action of minoxidil sulfate-induced vasodilation: a role for increased K+ permeability. J Pharmacol Exp Ther. 1988. 245:751–760.
19. Olsen EA. Olsen EA, editor. Androgenetic alopecia. Disorders of hair growth: diagnosis and treatment. 1994. New York: McGraw-Hill;257–283.
20. Yun SK, Kim HY, Ihm CW. Plasma levels of dehydroepiandrosterone sulfate (DHEA-S) and total testosterone in the patients with female androgenetic alopecia. Korean J Dermatol. 1995. 33:1060–1065.
21. Sawaya ME, Price VH. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol. 1997. 109:296–300.

22. Wozel G, Naranayan S, Jäckel A, Lutz GA. Alfatradiol (0,025%) - Eine wirksame und sichere Therapieoption zur Behandlung der androgenetischen Alopezie bei Frauen und Männern. Akt Dermatol. 2005. 31:553–560.

23. Orfanos CE, Vogels L. Local therapy of androgenetic alopecia with 17 alpha-estradiol. A controlled, randomized double-blind study (author's transl). Dermatologica. 1980. 161:124–132.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download