Abstract
Onychomycosis is usually caused by dermatophytes, but some nondermatophytic molds and yeasts are also associated with invasion of nails. Scopulariopsis brevicaulis is a nondermatophytic mold found in soil as a saprophyte. We report two cases of onychomycosis caused by S. brevicaulis in a 48-year-old male and a 79-year-old female. The two patients presented with a typical distal and lateral subungual onychomycosis. Direct microscopic examination of the potassium hydroxide preparation revealed fungal elements. From toenail lesions of the patients, brown colonies with powdery surface, which are a characteristic of S. brevicaulis, were cultured on two Sabouraud's dextrose agar plates. Three cultures taken from nail plates within a 2-week interval yielded similar findings. Numerous branched conidiophores with chains of rough walled, lemon-shaped conidia were observed in slide culture by light microscopy and scanning electron microscopy. The nucleotide sequences of the internal transcribed spacer for the two clinical isolates were identical to that of S. brevicaulis strain WM 04.498. To date, a total of 13 cases of S. brevicaulis onychomycosis including the two present cases have been reported in Korea. Mean age of the patients was 46.1 years, with a higher prevalence in males (69.2%). Toenail involvement was observed in all cases including a case involving both fingernail and toenail. The most frequent clinical presentation was distal and lateral subungual onychomycosis in 12 cases, while one case was proximal subungual onychomycosis.
Onychomycosis is mainly caused by dermatophytes, but is occasionally caused by nondermatophytic fungi including Scopulariopsis brevicaulis, Aspergillus spp., Fusarium spp. and Acremonium spp., which have been often considered as saprophytic or opportunistic fungi1. Since several cases of nondermatophytic onychomycosis have been reported, even in healthy subjects, recent increase in diseases with immune suppressed individuals as well as prominent environmental changes have brought these non-dermatophytic fungi into focus2,3. S. brevicaulis represents 1~10% of the non-dermatophytic onychomycoses3-5. Eleven cases of S. brevicaulis onychomycosis had been reported in Korea6,7.
Here, we report two additional cases of distal and lateral subungual onychomycosis (DLSO) diagnosed by clinical features, mycological culture, microscopic observations and molecular analysis, with a review of the literature on S. brevicaulis onychomycosis.
A 48-year-old male presented with a 1-year history of brownish-yellow discoloration with hyperkeratosis on the toenails. There was no history of other symptoms except for toenail dystrophy. Dermatological examination revealed the DLSO on the right toenails (1st, 2nd, 4th and 5th) and left toenails (1st and 5th) (Fig. 1). A 79-year-old female presented with yellow discoloration with hyperkeratosis on the right and left toenails during hospitalization due to herpes zoster. This patient had experienced progressive thickening and discoloration of distal and lateral nailplate (1st and 5th toenails of both feet) for 3 years without other symptoms.
Complete blood count, peripheral blood smear, urinalysis, liver and renal function tests and stool examination were within normal limits for both patients. Venereal disease research laboratory test and serological tests for hepatitis virus and human immunodeficiency virus were also negative. Chest x-ray and electrocardiogram were unremarkable. In mycological examination, fungal elements were observed in potassium hydroxide (KOH) preparations from the toenail lesions of both patients. Nail specimens were cultured on two slants of Sabouraud's dextrose agar (SDA) without cycloheximide at 25℃ for 2 weeks, which yielded several identical appearing colonies. But, there was no colony growth on slants of SDA containing cycloheximide. The moderately fast-growing colonies were initially white then turned buffy and powdery at their centers (Fig. 2). The back of each culture revealed a brownish tan center. We performed repeated (three) cultures taken from nail plates at 2-week intervals; all yielded similar findings. When the slide cultures of fungal colonies were stained with lactophenol cotton blue, numerous branched conidiophores with chains of conidia were observed by light microscopy (Fig. 3A). Structural features of rough-walled lemon-shaped conidia were observed in detail by scanning electron microscopy (Fig. 3B, C). The sequence analysis of internal transcribed spacer (ITS) 1 indicated that the two isolates were identical to S. brevicaulis strain WM 04.498 (GenBank accession number: AJ85377) (Fig. 4). From the light and scanning electron microscopy observation of the fungal culture in addition to the comparison of ITS 1 region, the isolates causing DLSO in two patients were identified as S. brevicaulis.
The male patient was successfully treated by 3 months administration of oral terbinafine (250 mg daily) and topical 5% amorolfine nail lacquer. At the follow-up, 9 months after the cessation of treatment, clinical and mycological recovery was confirmed. The female patient received oral terbinafine (250 mg daily) and topical 5% amorolfine nail lacquer for 4 weeks. However, this patient did not return for a therapeutic evaluation.
Onychomycoses comprising 50% of all onychopathies are caused mainly by dermatophytes and less frequently by non-dermatophytic molds and yeasts1. Non-dermatophytes such as Scopulariopsis spp., Aspergillus spp., Fusarium spp., and Acremonium spp. are responsible for 1.45~17.6% of fungal nail infections3-5. Among the genus Scopulariopsis, which are saprophytes found in soil worldwide, S. brevicaulis, S. brumptii, S. acremonium, S. fusca, and S. koningii are frequently related to human infections4,8. Most human infection caused by S. brevicaulis is onychomycosis, although there are several reports of other infections including skin infection, endocarditis, and endophthalmitis in patients with impaired immunity, trauma or surgery2,9,10. S. brevicaulis onychomycosis represents 1~10% of the nondermatophytic onychomycosis cases depending on the population, geographic regions, and the reporters3-5. These variations arise due to geographic differences in mold distribution, differences in the criteria used for the diagnosis of onychomycosis, and the use of different methods for fungal culture3. In Korea, its prevalence is lower, with reported rates of 1.41%6 and 1.23%11. S. brevicaulis onychomycosis is usually found in adults, commonly affecting toenails, because S. brevicaulis is a saprophytic mold found in soil12. As listed in Table 1, all 13 cases reported in Korea including the two present cases, were adults (mean age, 46.1 years) with higher prevalence in males (nine cases) than in females (four cases). All cases involved toenails, and, in one elderly male, involved both fingernails and toenails6,7.
Predisposing factors for nondermatophytic onychomycosis include footwear, hyperhidrosis, local trauma, family history, psoriasis, peripheral vascular disease, and immune suppression5. Among six patients whose history of other ailments was questioned, three cases presented with no underlying diseases, while the other three cases were associated with cerebral infarction, contusion, and hematoma (Table 1). However, the fact that one of two cases involving a 79-year-old female with onychomycosis for 3 years was hospitalized due to herpes zoster suggests that the patient might not have sustained an immunocompetent status.
Onychomycosis is classified into five clinical types: DLSO, proximal subungual onychomycosis (PSO), superficial white onychomycosis, endonyx onychomycosis, and dystrophic onychomycosis13. The most common type is DLSO, while PSO is relatively rare1. However, Tosti et al.3 observed 10 cases of PSO and seven cases of DLSO among 17 cases of S. brevicaulis onychomycosis. In Korea, DLSO has been the most common, with 12 cases, while one case involved PSO (Table 1)6,7.
Generally, dermatophytic infections are diagnosed by isolating the dermatophytes from the loci of infection, whereas the isolation of saprophytic S. brevicaulis may not necessarily indicate an infection14. Therefore, isolation of saprophytic molds and yeasts additionally requires microscopic observation of fungal elements such as hyphae and/or conidia from the lesions. To confirm the diagnosis, isolation and identification of the same species from repetitive cultures can be definitive15. In this report of two cases, fungal elements were found by microscopy, and the same fungal species were isolated from the repetitive cultures of toenail specimens. It may not be possible to distinguish the causative agents between S. brevicaulis and dermatophytes, which exhibit fragile nails with yellowish brown discoloration. Therefore, KOH examination and culture isolation are necessary for distinction. Thick-walled large conidiophores (4~9 µm) with chains of rough-walled, lemon-shaped conidia may be confirmed for the presumptive identification. When cultured, the fast-growing colonies are initially white then turn to buff and are powdery at the center. The back of the culture reveals a brownish tan center. When the slide cultures of fungal colonies were presently stained with lactophenol cotton blue, characteristic lemon-shaped conidia with small protrusions were observed in both cases by scanning electron microscopy (Fig. 3); similar observations have been noted8,10. Recently, sequencing analysis of the ITS 1 region of ribosomal RNA genes has been considered for the identification of the causative fungus of onychomycosis16. The ITS sequences were presently determined from the two isolates and then turned out to be identical to that of S. brevicaulis strain WM 04.498 (GenBank accession number AJ85377).
Treatment of onychomycosis has been difficult despite the development of new antifungals. The aims are, of course, the clinical and mycological cure of nails, but more than 25% of all patients reveal an incomplete or no response17. In particular, S. brevicaulis is difficult to eradicate from onychomycosis and deeper infections11,18. Nolting et al.19 reported that three cases out of six were cured by oral terbinafine (250 mg/day for 48 weeks). Tosti et al.18 in 1996 treated a case by intermittent itraconazole (400 mg/day for a week each month) for 4 months, but Tosti et al.3 in 2000 observed a 69.2% cure rate by chemical removal of nails with either topical terbinafine or 8% ciclopirox nail lacquer. De Doncker et al.20 reported that eight cases out of 10 were clinically and mycologically cured by intermittent administration of itraconazole. In Korea, the cure rates have been quite low: one case out of seven by intermittent itraconazole therapy6 and none of four cases due to incomplete therapy7. In this report of two cases, a male was successfully treated with oral terbinafine (250 mg daily) and topical 5% amorolfine nail lacquer for 3 months, while a female was administered with the same medication for only 4 weeks because she failed to revisit for therapeutic evaluation.
Identification of the causative agent is indispensible to select a proper treatment for onychomycosis, as untreated nondermatophytic molds including S. brevicaulis may develop into deeper or disseminated mycosis.
Figures and Tables
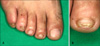 | Fig. 1(A) Brownish-yellow discoloration with hyperkeratosis on the toenails. (B) Close-up view of the right 2nd toenail. |
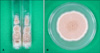 | Fig. 2Brown colonies with powdery surface after incubation at 25℃ for 2 weeks. (A) Sabouraud's dextrose agar slant. (B) Sabouraud's dextrose agar plate. |
 | Fig. 3(A) Slide culture of Scopulariopsis brevicaulis showing numerous branched conidiophores with chains of lemon-shaped conidia (Lactophenol-cotton blue stain, ×400). (B) Numerous branched conidiophores with chains of lemon-shaped conidia (scanning electron microscop [SEM], ×1,500). (C) Lemon-shaped conidia with a rough walled feature (SEM, ×3,000). |
References
1. Verma S, Heffernan MP. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Superficial fungal infection: dermatophytosis, onychomycosis, Tinea Nigra, Piedra. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1807–1821.
2. Bruynzeel I, Starink TM. Granulomatous skin infection caused by Scopulariopsis brevicaulis. J Am Acad Dermatol. 1998. 39:365–367.
3. Tosti A, Piraccini BM, Lorenzi S. Onychomycosis caused by nondermatophytic molds: clinical features and response to treatment of 59 cases. J Am Acad Dermatol. 2000. 42:217–224.

4. Gianni C, Cerri A, Crosti C. Non-dermatophytic onychomycosis. An understimated entity? A study of 51 cases. Mycoses. 2000. 43:29–33.

6. Kim SC, Chon TH, Kim HU. Onychomycosis due to Scopulariopsis brevicaulis. Korean J Dermatol. 2000. 38:1566–1568.
7. Kim YJ, Lim SW, Suh MK, Choi JH, Bang JS, Lee JW, et al. Four cases of toenail onychomycosis caused by Scopulariopsis brevicaulis. Korean J Med Mycol. 2001. 6:97–103.
8. Kwon-Chung KJ, Bennett JE. Medical mycology. 1992. Philadelphia: Lea & Febiger;752–755.
9. Oh BJ, Chae MJ, Cho D, Kee SJ, Shin MG, Shin JH, et al. Infection with Scopulariopsis brevicaulis after Cosmetic Surgery of the Face. Korean J Lab Med. 2006. 26:32–35.

10. De Hoog GS, Guarro J, Gene J, Figueras MJ. Atlas of clinical fungi. 2000. 2nd ed. Virgili: Centraalbureau voor Schimmelcultures;906–908.
11. Lim SW, Suh MK, Ha GY. Clinical features and identification of etiologic agents in onychomycosis (1999-2002). Korean J Dermatol. 2004. 42:53–60.
12. Onsberg P. Scopulariopsis brevicaulis in nails. Dermatologica. 1980. 161:259–264.
13. Baran R, Hay RJ, Tosti A, Haneke E. A new classification of onychomycosis. Br J Dermatol. 1998. 139:567–571.

14. Arrese JE, Piérard-Franchimont C, Piérard GE. Unusual mould infection of the human stratum corneum. J Med Vet Mycol. 1997. 35:225–227.

16. Makimura K, Tamura Y, Mochizuki T, Hasegawa A, Tajiri Y, Hanazawa R, et al. Phylogenetic classification and species identification of dermatophyte strains based on DNA sequences of nuclear ribosomal internal transcribed spacer 1 regions. J Clin Microbiol. 1999. 37:920–924.

17. Santos DA, Hamdan JS. In vitro antifungal oral drug and drug-combination activity against onychomycosis causative dermatophytes. Med Mycol. 2006. 44:357–362.

18. Tosti A, Piraccini BM, Stinchi C, Lorenzi S. Onychomycosis due to Scopulariopsis brevicaulis: clinical features and response to systemic antifungals. Br J Dermatol. 1996. 135:799–802.

19. Nolting S, Brautigam M, Weidinger G. Terbinafine in onychomycosis with involvement by non-dermatophytic fungi. Br J Dermatol. 1994. 130:Suppl 43. 16–21.

20. De Doncker PR, Scher RK, Baran RL, Decroix J, Degreef HJ, Roseeuw DI, et al. Itraconazole therapy is effective for pedal onychomycosis caused by some nondermatophyte molds and in mixed infection with dermatophytes and molds: a multicenter study with 36 patients. J Am Acad Dermatol. 1997. 36:173–177.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download