Abstract
Background
A stem cell is an undifferentiated cell that has the potential for self-renewal and differentiation. Adipose-derived stem cells (ADSCs) have advantages in accessibility and abundance compared to other kinds of stem cells and produce many growth factors and hormones.
Objective
We investigated whether ADSC cultured media could be used as a therapy for atopic dermatitis.
Methods
ADSC cultured media was topically applied twice daily for 5 days to oxazolone-treated atopic dermatitis-like hairless mice.
Results
Topical application of ADSC cultured media improved the epidermal permeability barrier and keratinocyte differentiation, and restored the predominant Th2 phenotype when compared to vehicle. ADSC cultured media-treated epidermis also showed an increase in the expression of antimicrobial peptides cathelin-related antimicrobial peptide, mouse beta-defensein 3.
Atopic dermatitis (AD) is a common, chronic inflammatory skin disorder characterized by pruritic skin lesions, immunodysregulation, disrupted epidermal barrier function, and immunoglobulin E (IgE)-mediated sensitization to food and environmental allergens. The striking increase in the incidence of AD observed in recent decades has been attributed to the resettlement of populations from rural to urban areas, where a lack of early exposure to a variety of microbes purportedly results in reduced immune tolerance. Long-term systemic therapy or combinations of multiple treatments are often used in the management of AD. These conventional approaches for the treatment of AD include systemic and topical anti-inflammatory, antipruritic, and immunosuppressive agents, as well as phototherapy1. However, no single agent is always effective for the treatment of AD. Therefore, many studies have sought to develop novel agents or methods for the management of AD.
The stem cell is an undifferentiated cell that has the potential for self-renewal and differentiation. Recently, adult stem cells have received attention because they are associated with fewer ethical difficulties than embryonic stem cells and less potential risk of carcinogenesis. Among adult stem cells, adipose-derived stem cells (ADSCs), mesenchymal stem cells that are extracted from human adipose tissue, have essentially the same properties as stem cells derived from bone marrow2. Moreover, ADSC have relative advantages in accessibility and abundance compared to other types of adult stem cells and produce many growth factors and hormones. Recently, several studies of the mechanism of action of stem cells such as anti-inflammatory and immunomodulatory actions have been reported3-5. Therefore, we investigated whether ADSC cultured media could be used as a novel therapeutic modality for AD.
As a model of AD, an epidermal hyperproliferative model (ideally also accompanied by dermal inflammation) would be preferred to assess the therapeutic efficacy and to study the action mechanisms responsible for the efficacy of ADSC cultured media. Therefore, this study employed an oxazolone-induced AD-like mouse model6 to assess the therapeutic efficacy of topical ADSC cultured media.
Twenty five female hairless mice (8 weeks old) were purchased from the animal laboratory of Yonsei University. Mice were kept under controlled humidity (40%) and temperature (22±2℃). 4-Ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone) and acetone were purchased from Sigma-Aldrich (St. Louis, MO, USA). All laboratory measurements were performed under blinded conditions or by blinded researchers. Study sample sizes were determined based on available reference data for epidermal permeability barriers7.
All animal procedures were approved by the Yonsei University Wonju Campus Institutional Animal Care and Use Committee. Development of a hapten (oxazolone)-induced, murine model with multiple features of AD (oxazolone-AD) was described in previous studies6. Each group (n=6 for each) of mice was sensitized by one topical treatment with 60 µl of 5% oxazolone, while the ethanol-treated vehicle group (n=6) served as the control (Fig. 1). After 13 days of multiple oxazolone challenges, all mice were evaluated the basal transepidermal water loss (TEWL), stratum corneum hydration and severity of erythema, and there is no statistical differences between each groups except the ethanol-treated vehicle group (data not shown).
Subcutaneous adipose tissue was obtained from healthy female human donors and washed three times with phosphate buffered saline (PBS) to remove debris and red blood cells. Washed aspirates were digested with 0.075% collagenase (Type 1; Sigma-Aldrich) for 45 min at 37℃ with constant shaking. Mature adipocytes and connective tissues were separated from the pellets by centrifugation at 1,200 rpm for 10 min. Pellets were resuspended in PBS, passed through a 100 µm mesh filter, and washed twice with PBS. The cell pellets containing ADSC were resuspended in low glucose Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco) and 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco), and plated at a density of 2~3×105 cells/cm2 in T75 flasks. Cultures were maintained at 37℃ in a humidified atmosphere containing 5% CO2. After 7~10 days, the cells were detached and remnant extract (media without cells) was used as ADSC cultured media.
Human dermal fibroblastic MRC-5 cells were obtained from American Type Culture Collection (No. CCL-171; Manassas, VA, USA) and used as controls, and cultured in the aforementioned supplemented DMEM.
After sensitization by oxazolone, 30 µl of ADSC cultured media were topically applied twice a daily with a micropipette on the dorsal skin ADSC cultured media applied (AM) group of each mouse for 10 days. For the control groups, minimal media applied (MM) group, fibroblast cultured media applied (FM) group and glucocorticosteroid (0.1% methylprednisolone aceponate cream, S) were topically applied (0.2 g twice daily). Twenty four hours after the last treatment, biopsy specimens were taken to evaluate changes in morphology and protein expression.
Both at baseline and at the end of the treatment period, basal TEWL was measured with a Tewameter TM210 electrolytic water analyzer (Courage and Khazaka, Cologne, Germany) and was stratum corneum (SC) hydration assessed as capacitance with a Corneometer CM820 (Courage and Khazaka) was measured immediately before each application of oxazolone and at 24 hours after the final application of oxazolone as described previously8.
Terminal differentiation marker proteins of keratinocytes (filaggrin, involucrin, and loricrin; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used for immune identification of epidermal differentiation. Briefly, 5 µm-thick paraffin sections were incubated with primary antibody overnight at 4℃. After three washes, sections were incubated with the secondary antibody for 30 min. Staining was detected with an ABC-peroxidase kit (Vector Lab, Burlingame, CA, USA), and sections were then counterstained with hematoxylin. To assess the immunohistochemistry of proliferating cells, changes in overall morphology were visualized after hematoxylin and eosin staining of 5 µm-thick paraffin-enabled sections, and proliferating cells were detected by proliferating cell nuclear antigen staining.
Immunofluorescence was used to evaluate changes in T helper 2 cell subpopulation expression. Five micrometer-thick frozen sections were incubated with rabbit anti-mouse Th2 immunophenotype (CRTH2)/DP2 antibody overnight at 4℃, followed by incubation with fluorescence conjugated goat anti-rabbit antibody for 30 min at room temperature. Both cathelin-related antimicrobial peptide C (CRAMP) (murine homolog of LL37) and mouse beta-detensin 3 mouse beta-defensin (mBD3) (murine homolog of human β-defensin 2) protein levels were assessed to determine whether antimicrobial peptide (AMP) expression changes were present in the epidermis, as described for human AD9. Slides were counterstained with propidium iodide and visualized on a TCS-SPE confocal microscope (Leica Systems GmbH, Watzlar, Germany) Controls without primary antibody showed no immunolabeling10.
To quantify the density of markers, three pictures for each section were taken at a 100x magnification and were labeled. An investigator without knowledge of the origin of the specimen ranked the pictures in order of the intensity of immunostaining (highest intensity: first rank, in descending order in each). Mann-Whitney test was used for statistics. A representative picture for each group was chosen for publication.
Murine dermal samples were homogenized in mammalian cell lysis buffer from a Qproteome Mammalian Protein Prep Kit (Qiagen, Valencia, CA, USA) containing Benzonase® (Merck, Darmstadt, Germany) nuclease and protease inhibitor solution. Lysates were centrifuged at 14,000 g for 10 min, and supernatants were collected to perform Western blots. Protein concentration was determined by a Bradford assay. Equal amounts of proteins were resolved by 10% SDS-polyacrylamide gel electrophoresis and electrophoretically transferred to polyvinylidene difluoride membranes. Membranes were subsequently blocked with 5% skim milk in TBST (20 mM Tris-HCl, pH 7.6, 137 mM NaCl, 0.1% Tween-20) and incubated with the indicated antibodies. For detection of CRAMP and mBD3, CRAMP antibody and mBD3 antibody were purchased from Santa Cruz Biotechnology. Blotting proteins were visualized by enhanced chemiluminescence (Amersham, Buckinghamshire, UK).
Total RNA was isolated from skin specimens using Trizol, then resuspended in RNase-free water and quantified with an ultraviolet spectrophotometer (Perkin Elmer, Waltham, MA, USA). Single-strand cDNA was prepared from 1 µg of total RNA in a 20 µl reaction volume using an Oligo-dT primer (Roche Molecular Systems, Branchburg, NJ, USA), which contained 5 mM MgCl2, 1 mM dNTP mixture, 1 U/µl RNase inhibitor (Roche Molecular Systems) and 0.25 U/µl M-MLV reverse transcriptase (Promega, Madison, WI, USA).
Real-time RT-PCR was performed on a Rotor-Gene™ 3000 (Corbett Life Science, NSW, Australia) using SYBR Green PCR Master Mix (Qiagen). All primers and probes were from Operon Biotechnologies (Huntsville, AL, USA). Primer and probe sequences for real-time RT-PCR analyses were: FAS primers (forward: 5'-CTGAAGAGCCTGGAAGATCG-3', reverse: 5'-TGTCACGT TGCC ATG G TACT-3') and FAS probe (5'-TGAGCTTTGCTG CCGTGTCC-3'); SPT primer (forward: 5'-GAGAGATGCTGAAGCGGAAC-3', reverse: 5'-TGGTATGAGCTGCTGACAGG-3') and SPT probe (5'-TGGGATTTCCTGCTACCCCG-3'). CRAMP and mBD-3 PCR products were quantitatively analyzed with densitometry and then normalized to β-actin.
We evaluated the effect of ADSC cultured media on the epidermal permeability barrier that had been disrupted by multiple oxazolone challenges. Basal TEWL was increased by oxazolone challenge compared with vehicle (C group) (Fig. 2A). But, after topical application of AM group (p<0.001) and steroids applied (S) group, p<0.005) TEWL was significantly lower than other treatments (FM and MM group). Furthermore, topical application of AM group, FM group, and S group improved the hydration of the stratum corneum compared with the MM group (Fig. 2B). Topical application of ADSC cultured media also improved visible lesions (Fig. 3). Skin that was treated with ADSC extracts in the absence of multiple oxazolone challenges showed no changes in barrier function (data not shown).
We next determined whether the oxazolone-induced murine model of AD displayed the Th2 dominant immunophenotype and whether abnormal keratinocyte differentiation was reversed. After repeated oxazolone challenges, lymphocyte-dominant infiltrates increased progressively with some lymphocytes appearing to invade the overlying epidermis. Most became CRTH2-positive, indicating a Th2 phenotype. Epidermal manifestations of filaggrin, loricrin, and involucrin, which represent the differentiation marker proteins of keratinocytes, decreased. In the AD-like model, topical application of ADSC cultured media resulted in a significant decrease in CRTH2 immunostaining, representing Th2 lymphocytes, and also resulted in a significant increase in the markers for keratinocyte differentiation (Fig. 4) compared with the MM and FM groups (p<0.05). In addition, there are no significant changes of keratinocyte differentiation that was treated with ADSC extracts in the absence of oxazolone challenges (data not shown).
The epidermal permeability barrier and AMP expression are co-regulated and have interrelated functions. We, therefore, assessed whether topical ADSC extracts reversed AMP expression, which was reduced by multiple oxazolone challenges. Immunohistochemical staining for mBD3 and immunofluorescent staining for CRAMP were carried out with the same mouse skin samples. There are no significant changes of AMP expressions that were treated with ADSC extracts in the absence of oxazolone challenges (data not shown). However, topical application of ADSC cultured media induced increased epidermal expression of AMP compared with other treatments (MM and FM groups) in the murine model of AD (Fig. 5). Immunohistochemical staining was confirmed by Western blot analysis, which showed that protein levels of mBD3 and CRAMP significantly increased after topical application of ADSC cultured media (Fig. 6A, p<0.05). The mRNA levels of mBD3 and CRAMP were quantified at the transcriptional level by real-time RT-PCR (Fig. 6B), and were significantly higher in the AM and S groups than in other treatment groups (MM and FM, p<0.05).
This study aimed to investigate the potential of ADSC cultured media to restore the epidermal permeability barrier and AMP expression, both of which were disrupted by multiple oxazolone challenges in an animal model of AD. Based on our results, we believe that topical ADSC cultured media can reduce the features of AD.
Stem cell therapy is becoming accepted as a new paradigm for the repair of organ function after damage by disease or trauma11. Embryonic stem cells obtained from human fetuses have been investigated, but barriers encountered include ethical concerns and potential for carcinogenesis. Adult stem cells are different from embryonic stem cells because they are obtained after the developmental process is completed and can be harvested after birth. In addition, adult stem cells have variable reproductive properties and potentials, which are like the characteristics of embryonic stem cells. Furthermore, adult stem cells have the possibility of self-transplantation 12. Mesenchymal stem cells, a subtype of undifferentiated adult stem cells, are present in differentiated tissues or organs. Among mesenchymal stem cells, ADSC have the relative advantages of accessibility and abundance. They are easily obtained from subcutaneous fat tissue with little pain to the patient. In addition, the possibility of an immunologic reaction is reduced because the cells originate from autologous stem cells. For these reasons, many recent experiments on stem cells have used ADSC. Here, we applied the cultured media of ADSC because it contains many proteins and cytokines released from ADSC.
In AD, decreased SC hydration leads to a steeper water gradient across the SC as a result of filaggrin deficiency, thereby 'driving' increased transcutaneous water loss. In addition, decreased generation of filaggrin products results in an initial increase in the SC pH, which can precipitate multiple downstream structural and functional alterations, including Th2 inflammation and degrading AMPs. Keratinocyte differentiation is also incomplete in AD lesions13. In the oxazolone-induced murine model of AD, permeability barrier function and expression of differentiation proteins like filaggrin, loricrin, and involucrin became abnormal. A CRTH-positive, Th2-dominant inflammatory infiltrate with increased IL-4 expression and a large increase in serum IgE levels were observed. Moreover, CRAMP and mBD3, as well as SC ceramide, declined after oxazolone challenges, paralleling the decrease of their human homologues in AD6. Therefore, we used an oxazolone-induced murine model of AD in this study to investigate the restoration of overall barrier function in AD.
In this study, we compared treatment with ADSC media to other treatments in order to determine whether ADSC media improves visible AD lesions, TEWL, SC hydration, and abnormal keratinocyte differentiation in an oxazolone-induced animal model. In our murine model of AD, the decreased overall skin barrier function and differentiation markers, involucrin, filaggrin, and loricrin, were improved after application of ADSC extracts compared with the vehicle-treated group. These findings suggest that topical ADSC cultured media has a barrier recovery function when applied to the skin lesions of AD.
Recently, some studies have reported on the anti-inflammatory effects of mesenchymal stem cells, which produce pro-inflammatory cytokines like transforming growth factor-beta (TGF-β), hepatocyte growth factor (HGF), interleukin (IL)-4, IL-10, and prostaglandins, or some combination thereof, and suppress the cytokines/chemokines involved in activation, differentiation, and recruitment of immunoregulatory cells such as neutrophils, basophils, B cells, T cells, granulocytes, and cells noted for exacerbating inflammation such as monocytes/macrophages3,4. In our study, we confirmed that cultured media of ADSC has an anti-inflammatory effect on an oxazolone induced murine model of AD compared with other treatments, including control media. Therefore, we believe that ADSC cultured media contains anti-inflammatory cytokines and/or chemokines released from ADSC, and that these products are a mechanism for relieving inflammation and restoring Th2 dominant lymphocytes in the dermis.
AMPs contribute to the establishment of adaptive immune reactions and inflammation and also play a major role in innate immunity to defend against microbes14. Although decreased expression of AMP is related to increased susceptibility to cutaneous infections in atopic skin, excessive and uncontrolled expression of AMP may be involved in the induction and propagation of inflammation in psoriatic skin14. Thus, as dysregulated expression of AMP may be important for the pathogenesis of various chronic inflammatory diseases, controlling and balancing AMP expression may offer a novel therapeutic approach15. Human mesenchymal stem cells have direct antimicrobial activity, which is mediated in part by the secretion of human cathelicidin hCAP-18/LL-3716. Given these studies and our results, ADSC cultured media may play a major role in balancing the expression of epidermal AMP.
However, there are some limitations to the effectiveness of ADSC cultured media. The concentration of ADSC cultured media depends on the general status of its donor, such as age, sex, and medical status. Therefore, it is difficult to maintain a standardized concentration and content. This limitation is similar to that of herbal medicine, but can be corrected to some degree by selecting standardized donors. Moreover, previous studies have produced inconsistent results with the components of cytokine in stem cell extracts, which showed only prostaglandins and not IL-10, TGF-β, or HGF3-5. Therefore, an advanced and standardized cytokine profile assay may be indispensible for analyzing the cytokines of stem cell extracts in further studies.
In summary, we confirm that ADSC cultured media improves visible AD lesions, TEWL, SC hydration, keratinocyte differentiation, AMPs, and inflammation in an oxazolone-induced murine model of AD. From these results, we conclude that topical application of ADSC cultured media improves AD lesions through a mechanism improving the epidermal permeability barrier, differentiation, and epidermal AMP expression, as well as suppressing TH2 cells. This result indicates that ADSC cultured media may be useful in the treatment of AD.
Figures and Tables
 | Fig. 1Methods of multiple oxazolone (Ox) challenges and topical administration of adipose-derived stem cell cultured media. |
 | Fig. 2(A) Basal TEWL (gm-2h-1) was checked after the fifth challenge of oxazolone (MM-pre, AM-pre, FM-pre, S-pre). The AM group and S group showed significant decreases in TEWL after treatment (C-post, MM-post, AM-post, FM-post and S-post). (B) SC hydration after treatment. AM, FM, and S groups showed significant improvement of SC hydration. Data are expressed as mean±standard deviation; n=6 in MM, AM, and FM group; n=4 in C group; n=3 in S group. TEWL: transepidermal water loss, SC: stratum corneum, C: control group, MM: minimal media applied group, AM: adipose-derived stem cell cultured media applied group, FM: fibroblast cultured media applied group, S: steroid applied group. |
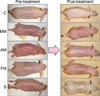 | Fig. 3Gross lesions of pre- and post-treatment with media in the atopic dermatitis model. The AM group and S group showed an improvement in skin lesions grossly. C: control group, MM: minimal media applied group, AM: adipose-derived stem cell cultured media applied group, FM: fibroblast cultured media applied group, S: steroid applied group. |
 | Fig. 4Th2 immunophenotype (CRTH2) and keratinocyte differentiation (filaggrin, involucrin, and loricrin) after treatment. Immunohistochemical staining was carried out with diaminobenzidine and primary antibodies as indicated. Merged images with propidium iodide secondary staining in 5 mm-thick frozen sections. C: control group, MM: minimal media applied group, AM: adipose-derived stem cell cultured media applied group, FM: fibroblast cultured media applied group, S: steroid applied group (Mag bars=2 mm). |
 | Fig. 5Topical application of adipose-derived stem cell cultured media enhanced the epidermal expression of antimicrobial peptides such as mBD3 and CRAMP. Immunohistochemical staining was carried out with diaminobenzidine and primary antibodies as indicated. mBD3: mouse beta-defensin 3, CRAMP: cathelin-related antimicrobial peptide, C: control group, MM: minimal media applied group, AM: adipose-derived stem cell cultured media applied group, FM: fibroblast cultured media applied group, S: steroid applied group (Mag bars=2 mm). |
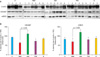 | Fig. 6Topical application of adipose-derived stem cell cultured media increased the protein and mRNA levels of mBD3 and CRAMP compared with other treatments in Western blots (A) and for mRNA of mBD3 and CRAMP compared with vehicle in real-time reverse transcriptase-polymerase chain reaction (B). This is compatible with immunohistochemical staining results. Data are expressed as mean±standard deviation; n=6 in MM, AM and FM group; n=4 in C group; n=3 in S group. CRAMP: cathelin-related antimicrobial peptide; mBD3: mouse beta-defensin 3, C: control group, MM: minimal media applied group, AM: adipose-derived stem cell cultured media applied group, FM: fibroblast cultured media applied group, S: steroid applied group. |
ACKNOWLEDGMENT
This work was supported by a research grant from Yonsei University Wonju College of Medicine (YUWCM 2012-51).
References
1. O'Regan GM, Irvine AD. The role of filaggrin in the atopic diathesis. Clin Exp Allergy. 2010. 40:965–972.
2. Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006. 24:1294–1301.

3. Ripoll CB, Flaat M, Klopf-Eiermann J, Fisher-Perkins JM, Trygg CB, Scruggs BA, et al. Mesenchymal lineage stem cells have pronounced anti-inflammatory effects in the twitcher mouse model of Krabbe's disease. Stem Cells. 2011. 29:67–77.

4. Razmkhah M, Jaberipour M, Erfani N, Habibagahi M, Talei AR, Ghaderi A. Adipose derived stem cells (ASCs) isolated from breast cancer tissue express IL-4, IL-10 and TGF-β1 and upregulate expression of regulatory molecules on T cells: do they protect breast cancer cells from the immune response? Cell Immunol. 2011. 266:116–122.

5. Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Weimar W, Hoogduijn MJ. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev. 2010. 19:1843–1853.

6. Man MQ, Hatano Y, Lee SH, Man M, Chang S, Feingold KR, et al. Characterization of a hapten-induced, murine model with multiple features of atopic dermatitis: structural, immunologic, and biochemical changes following single versus multiple oxazolone challenges. J Invest Dermatol. 2008. 128:79–86.

7. Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008. 128:917–925.

8. Choi EH, Brown BE, Crumrine D, Chang S, Man MQ, Elias PM, et al. Mechanisms by which psychologic stress alters cutaneous permeability barrier homeostasis and stratum corneum integrity. J Invest Dermatol. 2005. 124:587–595.

9. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002. 347:1151–1160.

10. Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007. 117:3339–3349.

11. Meyer GP, Wollert KC, Drexler H. Stem cell therapy: a new perspective in the treatment of patients with acute myocardial infarction. Eur J Med Res. 2006. 11:439–446.
12. Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997. 275:964–967.

13. Elias PM. Therapeutic Implications of a Barrier-based Pathogenesis of Atopic Dermatitis. Ann Dermatol. 2010. 22:245–254.

14. Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009. 30:131–141.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download