Abstract
Background
Pruritis caused by atopic dermatitis (AD) is not always well controlled by topical corticosteroid therapy, but use of tacrolimus often helps to soothe such intractable pruritis in clinical settings.
Objective
To determine the anti-pruritic efficacy of topical tacrolimus in treating AD in induction and maintenance therapy.
Methods
Prior to the study, patients were randomly allocated into two groups, induction therapy followed by tacrolimus monotherapy maintenance, and induction therapy followed by emollient-only maintenance. In the induction therapy, the patients were allowed to use topical tacrolimus and emollients in addition to a low dose (<10 g/week) of topical steroids. Patients showing relief from pruritis were allowed to proceed to maintenance therapy. Recurrence of pruritis in maintenance therapy was examined as a major endpoint.
Atopic dermatitis (AD) is a common chronic or chronically relapsing, severely pruritic, and eczematous skin disease whose prevalence appears to have increased significantly in recent decades1,2. The control of pruritis, a primary symptom of AD, is very important in its treatment since pruritis itself is an unpleasant sensation that often disturbs patients' sleep. Additionally, incidental scratching exacerbates and sustains skin eruptions, thereby significantly reducing patient quality of life. However, pruritis caused by AD is not readily controlled with clinically available oral antihistamines, probably due to the presence of many inflammatory pruritogenic factors other than histamine3-5. Thus, one of the simplest and most practical answers is to reduce or eliminate skin inflammation by the use of strong anti-inflammatory agents such as topical corticosteroids. Indeed, this strategy is effective in most cases in treating pruritis as well as skin inflammations caused by AD6. However, there are substantial numbers of patients undergoing topical corticosteroid therapy who still suffer from intractable pruritis and whose extensive scratching aggravates their dermatitis. Calcineurin inhibitors are a relatively new treatment for AD, and orally administered cyclosporine has been reported effective in treating refractory pruritis in patients with AD7. Similarly, the anti-pruritic effects of topical calcineurin inhibitors have also been reported8. Thus, the purpose of this study was to further evaluate the anti-pruritic efficacy of topical tacrolimus, a calcineurin inhibitor, in the treatment of patients with AD in inductive and maintenance treatment.
Patients with AD who were >10 years old and whose visual analogue scale (VAS)-itch scores (max=100) were 30~80 were recruited after written informed consent was obtained. Patients whose VAS-itch scores were >80 were excluded because of their desperate need for anti-pruritic treatment including antihistamines or more potent systemic anti-inflammatory treatment. Conversely, patients whose VAS-itch scores were <30 were excluded because of their lesser need for additional anti-pruritic therapy and the limited window in assessing pruritis improvement. Patients who had been treated with orally administered corticosteroids, cyclosporine, or antihistamines within two weeks prior to the registration were also excluded because of their potential influence on pruritis.
All of the patients received induction (1~4 weeks) and maintenance (>4 weeks) therapy. Prior to the study, patients were randomly allocated in advance into two groups: patients who received topical tacrolimus monotherapy as maintenance therapy after induction therapy and patients who received emollient only for maintenance therapy after induction therapy. In the induction therapy, all of the patients were treated with topical tacrolimus (of 0.03% for patients <16 years old and of 0.1% otherwise) and emollients twice daily in addition to their usual topical corticosteroid treatment (maximum use, 10 g/week), and change of VAS-itch score was examined. Patients who showed a reduced VAS-itch score by >20 points were considered to show relief from pruritis, while only such induction therapy responders proceeded into maintenance treatment. In maintenance therapy, recurrence of pruritis, mean change of VAS-itch scores, and the percentage of patients with pruritis recurrence were measured. Patients who showed increased VAS-itch scores of >20 points were categorized as suffering from pruritis recurrence in maintenance treatment. Secondarily, skin severity score was monitored using the SCORing Atopic Dermatitis (SCORAD) score9. This study was an open label, randomized, multi-center study and was approved by the internal ethical review boards of Kyushu University and other institutions.
The confidence interval (CI) for the proportion of subjects who experienced pruritis relief was estimated in the induction therapy using Fisher's exact method assuming a binomial distribution, while changes in VAS-itch score and SCORAD were assessed using the paired t-test. The cumulative proportion of pruritis recurrence was estimated using the Kaplan-Meier method, while the CI was estimated using Greenwood's method in maintenance treatment. The percentage difference in pruritis recurrence between the two groups was assessed using Fisher's exact test. The mean difference between VAS-itch score and its 95% CI were estimated using analysis of covariance (ANCOVA). The time elapsed before pruritis recurrence was assessed using the stratified log-rank test, with institutions divided into "Kyushu University," "University of Tokyo," and "other institutions."
A total of 70 patients with AD were registered, 68 of whom completed induction therapy (Fig. 1). A total of 44 of the 68 patients experienced pruritis relief (64.7%; 95% CI, 53.1~76.4%), while 43 of the 44 responders proceeded to maintenance therapy. The median and mean (standard deviation, SD) of the induction therapy period among the 43 patients were 15 days and 17.9 (7.1) days, respectively. Twenty-one patients each in the tacrolimus monotherapy group and the emollient group completed the maintenance treatment. No marked bias was apparent in the baseline data of the patients who were registered compared to those who completed this whole study (Table 1).
In the induction therapy, mean VAS-itch score (SD) decreased from 51.1 (16.6) to 32.3 (22.1) (Fig. 2A), while the mean difference, 18.8 (95% CI, 13.5~24.1), was statistically significant (p<0.0001). There was no statistical difference in mean VAS-itch score between responders and non-responders before the induction therapy (Fig. 2B), but there was a significant decrease of VAS-itch in responders after treatment (Fig. 2C). Data on disease severity (SCORAD) after the induction therapy were obtained from 50 of the 68 subjects who completed treatment (43 of the 44 pruritis-responders and 7 of the 24 non-responders). Mean SCORAD (SD) of the 50 subjects decreased from 29.1 (11.1) to 17.3 (10.6) (Fig. 2D), and the mean difference of 11.8 (95% CI, 9.0~14.7) was statistically significant (p<0.0001).
Cumulative itch recurrence in the tacrolimus monotherapy maintenance group and emollient maintenance group at day 28 was 23.8% (95% CI, 10.7~52.9%) and 100%, respectively in maintenance treatment (Fig. 3), and the difference between the two groups was statistically significant (p<0.0001). The median time to pruritis recurrence in the tacrolimus monotherapy group and the emollient group was >28 days and 3 days (95% CI, 2~5 days), respectively (Table 2). The mean VAS-itch score in the tacrolimus monotherapy group was well controlled as shown by values of 28.1 (15.4) at the start and 29.6 (20.9) at the end of maintenance treatment, while that in the emollient group significantly increased from 19.3 (16.7) to 50.7 (17.0) (Table 3). The mean change in VAS-itch scores was 1.50 (3.30) in the tacrolimus monotherapy and 31.4 (2.59) in the emollient group, respectively, in maintenance treatment (Fig. 4), and the difference, 28.6 (95% CI, 19.8~37.5), was statistically significant (p<0.0001).
A transient burning sensation by topical tacrolimus, the only distinguished side effect, was recorded in 32 of 69 patients (46.3%, excluding one dropout patient who never returned after initial registration) in the induction therapy. The other minor side effect was acne/folliculitis (3 cases, 4.3%), herpes simplex (1 case, 1.4%), wart (1 case, 1.4%), and the common cold (2 cases, 2.9%) throughout the study period.
In the induction therapy of this study, patients were allowed to use topical tacrolimus and emollients in addition to low-dose application (<10 g/week) of topical steroids. Almost two-thirds of the patients with AD experienced pruritis relief after the induction therapy. In these responsive patients, sequential maintenance by topical tacrolimus monotherapy was found to be significantly effective in controlling pruritis caused by AD compared with emollient only.
Orally administered cyclosporine appeared to effectively treat intractable pruritis in patients with AD as previously mentioned7. However, various adverse effects, such as systemic immune suppression, hypertension, headache and possible renal failure, should be considered and carefully monitored before and during administration. Topical tacrolimus, on the other hand, can basically avoid all of these undesirable adverse effects and is therefore more suitable for use in daily clinics, except for possible local immune suppression of the skin. However, one of our earlier studies and another report showed that topical tacrolimus was not associated with an increase in cutaneous infection10,11. Ultraviolet therapy is another option for treating intractable pruritis in patients with AD12, but it carries the possible risk of developing skin cancer in the long run13, raising a concern about its use, particularly in infants.
Hon et al.14 evaluated the clinical efficacy of topical tacrolimus for reducing the sensation of pruritis in children with AD. Three boys and four girls with AD were treated with topical tacrolimus for a consecutive two-week period after a one-week run-in. Nocturnal scratching activity measured using a DigiTrac movement recorder was reduced from 115.0 g/min to 71.5 g/min (p=0.028) after two weeks of treatment.
Such anti-pruritic effects of topical tacrolimus are thought to be due to its anti-inflammatory action considering the fact that the efficacy of 0.1% tacrolimus ointment was similar to that of 0.1% hydrocortisone butyrate ointment or 0.12% betamethasone valerate ointment when applied for three weeks in adults15,16. However, pruritis is not always readily relieved even after topical application of more potent corticosteroids in clinical settings. Several unique characteristics of tacrolimus that appear to be related to its anti-pruritic effects, such as inhibition of the epidermal sensory nerve extension17, transient release of substance P from sensory nerve endings18, and suppression of mast cell degranulation19 have been reported. There is currently no conclusive answer as to what governs the mechanism of anti-pruritic action of tacrolimus; however, one important fact is that topical tacrolimus does have an anti-pruritic property that topical corticosteroids lack17. We reproduced these results in a very similar experimental setting and extended the findings that the curious anti-pruritic effects of tacrolimus might not be simply due to its anti-inflammatory effects or anti-epidermal nerve extension effects20. In this study, the treatment responders (patients with decreased pruritis) showed a better change in SCORAD (disease severity) than non-responders in the induction therapy when data available were analyzed by ANCOVA (Fig. 5). However, we cannot determine from this whether the improved disease severity might come from less itching/scratching by the direct action of topical tacrolimus or that improved disease severity resulted in less production of various inflammatory pruritogens to bring about less pruritis. Anyhow, controlling itching/scratching is important in the formation of allergic skin reaction21, and further investigations will be needed to precisely identify the mechanism of action of anti-pruritic effects by anti-inflammatory agents.
Finally, this is an open study; therefore the possibility of a placebo effect (no exact vehicle control was used) should also be taken into consideration, such as possible anti-pruritic effects by the vehicle as an emollient. However, the use of emollients was allowed in both the tacrolimus monotherapy group and emollient group in maintenance treatment, thereby lessening this possibility.
In conclusion, topical tacrolimus is well tolerated (68/70 patients were able to complete the induction therapy) and significantly effective in controlling intractable pruritis during induction and maintenance therapy for patients with AD.
Figures and Tables
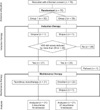 | Fig. 1Flow diagram showing subjects' progress. Patients were advance-allocated after registration, received introduction therapy (add-on tacrolimus therapy), and the responders to the introduction therapy proceeded into maintenance therapy. There were several dropouts and one refusal during the study. VAS: visual analogue scale. |
 | Fig. 2Change in visual analogue scale (VAS)-itch score and disease severity after add-on tacrolimus therapy. (A) Pruritis (mean VAS-itch score - standard deviation) reduced after add-on topical tacrolimus therapy. (B) There was no statistical difference in mean VAS-itch score between responders and non-responders before the add-on therapy. (C) There was a significant decrease in VAS-itch score in responders after the add-on therapy. (D) SCORing Atopic Dermatitis (SCORAD) score reduced after the add-on topical tacrolimus therapy. |
 | Fig. 3Cumulative recurrence of pruritis in maintenance therapy. Tacrolimus monotherapy group (solid line) showed significantly much lower recurrence of pruritis compared to that of the emollient group (dotted line). |
 | Fig. 4Efficacy of tacrolimus monotherapy in maintenance therapy. The emollient group showed more pruritis than the tacrolimus monotherapy group at the end of maintenance therapy. VAS: visual analogue scale. |
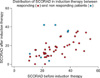 | Fig. 5Change of SCORing Atopic Dermatitis (SCORAD) in induction therapy between treatment-responding (blue circle) and non-responding patients (red circle) in induction therapy. Forty-three treatment-responding patients showed significantly reduced SCORAD compared to that of 7 non-responding patients in induction therapy as assessed by analysis of covariance (p=0.001). |
ACKNOWLEDGMENT
This work was supported by research grants from the Ministry of Health, Labour and Welfare, Japan. We thank Drs. Hiromichi Kai and Toyoaki Kato at the Department of Dermatology, University of Tokyo, for their outstanding help.
References
1. Takeuchi M, Ueda H. Increase of adult atopic dermatitis (AD) in recent Japan. Environ Dermatol. 2000. 7:133–136.
2. Yamamoto S. Prevalence and exacerbation factors of atopic dermatitis. Skin Allergy Frontier. 2003. 1:85–90.
3. Ikoma A, Steinhoff M, Ständer S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006. 7:535–547.

4. Rossi R, Johansson O. Cutaneous innervation and the role of neuronal peptides in cutaneous inflammation: a minireview. Eur J Dermatol. 1998. 8:299–306.
5. Ständer S, Steinhoff M, Schmelz M, Weisshaar E, Metze D, Luger T. Neurophysiology of pruritus: cutaneous elicitation of itch. Arch Dermatol. 2003. 139:1463–1470.
6. Furue M, Terao H, Rikihisa W, Urabe K, Kinukawa N, Nose Y, et al. Clinical dose and adverse effects of topical steroids in daily management of atopic dermatitis. Br J Dermatol. 2003. 148:128–133.

7. Wahlgren CF. Itch and atopic dermatitis: clinical and experimental studies. Acta Derm Venereol Suppl (Stockh). 1991. 165:1–53.
8. Boguniewicz M, Fiedler VC, Raimer S, Lawrence ID, Leung DY, Hanifin JM. Pediatric Tacrolimus Study Group. A randomized, vehicle-controlled trial of tacrolimus ointment for treatment of atopic dermatitis in children. J Allergy Clin Immunol. 1998. 102:637–644.

9. Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993. 186:23–31.
10. Furue M, Terao H, Moroi Y, Koga T, Kubota Y, Nakayama J, et al. Dosage and adverse effects of topical tacrolimus and steroids in daily management of atopic dermatitis. J Dermatol. 2004. 31:277–283.

11. Fleischer AB Jr, Ling M, Eichenfield L, Satoi Y, Jaracz E, Rico MJ, et al. Tacrolimus ointment for the treatment of atopic dermatitis is not associated with an increase in cutaneous infections. J Am Acad Dermatol. 2002. 47:562–570.

13. Chuang TY, Heinrich LA, Schultz MD, Reizner GT, Kumm RC, Cripps DJ. PUVA and skin cancer. A historical cohort study on 492 patients. J Am Acad Dermatol. 1992. 26:173–177.
14. Hon KL, Lam MC, Leung TF, Chow CM, Wong E, Leung AK. Assessing itch in children with atopic dermatitis treated with tacrolimus: objective versus subjective assessment. Adv Ther. 2007. 24:23–28.

15. Reitamo S, Rustin M, Ruzicka T, Cambazard F, Kalimo K, Friedmann PS, et al. European Tacrolimus Ointment Study Group. Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone butyrate ointment in adult patients with atopic dermatitis. J Allergy Clin Immunol. 2002. 109:547–555.

16. Japanese FK-506 Ointment Study Group. Phase III study of FK-506 (Tacrolimus) ointment in patients with atopic dermatitis -Comparison study with 0.12% betamethasone valerate ointment for trunk and extremities lesions-. Nishinihon J Dermatol. 1997. 59:870–879. (article in Japanese).
17. Inagaki N, Shiraishi N, Igeta K, Itoh T, Chikumoto T, Nagao M, et al. Inhibition of scratching behavior associated with allergic dermatitis in mice by tacrolimus, but not by dexamethasone. Eur J Pharmacol. 2006. 546:189–196.

18. Inagaki N, Shiraishi N, Igeta K, Nagao M, Kim JF, Chikumoto T, et al. Depletion of substance P, a mechanism for inhibition of mouse scratching behavior by tacrolimus. Eur J Pharmacol. 2010. 626:283–289.

19. Inoue T, Katoh N, Kishimoto S. Prolonged topical application of tacrolimus inhibits immediate hypersensitivity reactions by reducing degranulation of mast cells. Acta Derm Venereol. 2006. 86:13–16.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download