Abstract
Background
Adipose-derived stem cells (ASCs) are mesenchymal stem cells that have recently been applied to tissue repair and regeneration. Keratinocytes and dermal fibroblasts play key roles in cutaneous wound healing.
Objective
We investigated the paracrine effects of ASCs on HaCaT cells (i.e., immortalized human keratinocytes) and human dermal fibroblasts to explore the mechanism of the effects of ASCs on cutaneous wound healing.
Methods
HaCaT cells and primary cultured human dermal fibroblasts were treated with 50% conditioned medium of ASCs (ASC-CM). Viability, in vitro wound healing, and fibroblast-populated collagen lattice contraction assays were conducted, and reverse transcription-polymerase chain reaction (RT-PCR) for the type I procollagen α1 chain gene was performed.
Results
The proliferation of HaCaT cells and fibroblasts was increased by ASC-CM in the viability assay. ASC-CM promoted in vitro wound healing of HaCaT cells and increased the contraction of the fibroblast-populated collagen lattice. RT-PCR showed that the transcription of the type I procollagen α1 chain gene in fibroblasts was upregulated by ASC-CM.
Wound healing is a complex chain of events involving interactions among different cells and tissues. The progression of the wound healing process is impaired in a number of medical conditions1. Although new therapeutic methods have been developed, the treatment of many chronic wounds remains unsatisfactory, and more effective treatment strategies are needed.
Bone marrow-derived mesenchymal stem cells (BMSCs) are multipotent stem cells capable of differentiating into numerous cell types, including osteoblasts, chondrocytes, and adipocytes2-4. BMSCs can accelerate wound healing5,6, but BMSC transplantation requires the harvesting of a large amount of bone marrow under general anesthesia, which may lead to severe complications.
Adipose-derived stem cells (ASCs) represent an alternative source of multipotent cells with characteristics similar to BMSCs7,8. ASCs are easier to isolate and are relatively abundant, which may make them a better stem cell source for wound repair and regeneration. Although ASCs combined with atelocollagen matrix improves wound healing in healing-impaired db/db mice9, the effects of ASCs on cutaneous wound healing have not been clearly demonstrated.
In this study, we investigated the effects of conditioned medium of ASCs (ASC-CM) on HaCaT cells (i.e., immortalized human keratinocytes) and human dermal fibroblasts to explore the mechanism of action of ASCs in cutaneous wound healing.
All experimental protocols were approved by the Institutional Review Board of the Seoul National University Hospital.
Human subcutaneous adipose tissues were acquired from elective liposuction from healthy female donors with informed consent. The suctioned fat was digested with 0.075% collagenase type I (Sigma-Aldrich, St. Louis, MO, USA) in phosphate-buffered saline (PBS) under gentle agitation for 30 minutes at 37℃, and centrifuged at 800× g for 10 minutes to obtain the stromal cell fraction. The pellets were resuspended, passed through a 100-mm mesh filter (Millipore, Billerica, MA, USA), and cultured at 37℃ in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 mg/ml streptomycin, and 100 U/ml penicillin. After 3 days, the culture medium was changed to MesenPRO RS™ Medium (Invitrogen, Carlsbad, CA, USA), and the medium was replaced every 3 days. The primary cells were cultured until 80% confluence and were defined as passage 1. For the experiment, ASCs were used at passage 4.
Dermal fibroblasts were harvested from foreskin obtained from donors under informed consent and cultured as previously described10. Fibroblasts at passages 4~6 were used for the experiment. HaCaT cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 mg/ml streptomycin, and 100 U/ml penicillin at 37℃ in 5% CO2/95% air.
Cultured ASCs at passage 4 were characterized by immunostaining with antibody, using anti-CD29, anti-CD44 (Dako, Glostrup, Denmark), anti-CD31 (Novocastra, Newcastle, UK), and anti-CD34 (Becton Dickinson, Franklin Lakes, NJ, USA).
Capacities to differentiate along adiopogenic and osteogenic lineages were examined. Cell differentiation was initiated when ASCs at passage 4 were confluent in 35 mm-diameter culture dishes. For adipogenic differentiation, ASCs were incubated for 3 weeks in the culture medium supplemented with 0.5 mM isobutylmethylxanthine (Sigma-Aldrich), 1 µM dexamethasone, 10 µM insulin, and 200 µM indomethacin. Fixed cells (4% paraformaldehyde for 10 minutes) were washed with 60% isopropanol and incubated for 15 minutes with Oil red O to visualize lipid droplets. For osteogenic differentiation, cells were incubated for 3 weeks in the culture medium supplemented with 0.1 µM dexamethasone, 50 µM ascorbate-2-phosphate, and 10 mM β-glycerophosphate. Alkaline phosphatase staining was performed according to the manufacturer's instructions (BCIP/NBT color development substrate; Promega, Madison, MI, USA). Calcium deposits were detected by Alizarin red S staining.
Eighty percent confluent ASCs at passage 4 in 100 mm-diameter culture dishes were fed with 5 ml of serum-free DMEM and incubated at 37℃ in an atmosphere of 5% CO2/95% air for 48 hours. At the end of the incubation period, the media were collected and centrifuged at 300× g for 10 minutes to avoid contamination of cell fragments, and the supernatant was used as ASC-CM.
Cell proliferation was assessed using the MTT assay. Briefly, HaCaT cells and dermal fibroblasts were seeded in wells of 24-well culture plates at 5×103 cells/well and incubated at 37℃ in an atmosphere of 5% CO2/95% air. The next day, the cells were fed with serum-free DMEM for 24 hours, and then treated with serum-free DMEM (control medium) or 50% ASC-CM for 4 days. After removing the medium from each well, 300 µl of 0.5 mg/ml MTT solution was added and incubated for 3 hours at 37℃ in the dark. The supernatant was removed, and formazan crystals were dissolved in 200 µl of dimethyl sulfoxide. The dimethyl sulfoxide solution was transferred into 96-well plates and the optical density was measured at 570 nm using a spectrophotometer.
Confluent HaCaT cells in six-well culture plates were fed with serum-free DMEM. After 24 hours, the monolayer was scratched with a sterile plastic micropipette tip. The cells were washed three times with PBS, and serum-free DMEM (control medium) or 50% ASC-CM was added. Photographs of the wounds were taken at 0, 24, and 48 hours by phase-contrast microscopy, and the width of healed area was measured by the Image J analysis program (National Institutes of Health, Bethesda, MD, USA).
Five milliliters of bovine serum albumin (Sigma-Aldrich) solution was pipetted into each well of a six-well plate and incubated at 37℃ for 1 hour. Collagen solution mixture was prepared by quickly mixing eight volumes of collagen type I solution (Nitta Gelatin, Tokyo, Japan) with one volume of 10-fold concentrated DMEM and one volume of sodium bicarbonate (22 mg/ml). Dermal fibroblasts were added to the collagen solution mixture at a concentration of 3×105 cells/ml and gently mixed. Collagen solution mixture with fibroblasts (1.5 ml) was poured into each well of a 6-well plate after bovine serum albumin solution was removed, and allowed to gel for 30 minutes in 5% CO2/95% air at 37℃. The fibroblast-populated collagen lattices were detached from the surface of the well by rimming the lattices with a sterile needle and gently swirling the plate. Serum-free DMEM (control medium) or 50% ASC-CM was added to each well. After 5 days, digital photographs were taken and the size of the collagen lattice was measured by the Image J analysis program.
The expression of type I procollagen α1 chain gene in dermal fibroblasts was assessed by RT-PCR. Dermal fibroblasts were cultured in serum-free DMEM or 50% ASC-CM for 6 hours. Total RNA was isolated using TRIzol reagent (Invitrogen), and cDNA was synthesized with a cDNA synthesis kit (Promega). For PCR amplification, a 20-µl reaction mixture containing 1 U of Taq DNA polymerase, 250 µM dNTP, 10 mM Tris-HCl (pH 9.0), 40 mM KCl, and 1.5 mM MgCl2 was used. To ensure that a similar amount of cDNA was used for PCR, samples were also assessed for expression of h36B4 as a standard housekeeping gene. Nucleotide sequences of the PCR primers were as follows: type I procollagen α1 chain sense: CTC GAG GTG GAC ACC ACC CT, antisense: CAG CTG GAT GGC CAC ATC GG: h36B4 sense: TCG ACA ATG GCA GCA TCT AC, antisense: TGA TGC AAC AGT TGG GTA GC. Thermal cycling consisted of denaturation for 1 minute at 90℃, annealing for 1 minute at 60℃ and extension for 2 minutes at 72℃. The PCR program consisted of amplification cycles for type I procollagen α1 chain and 25 for h36B4. After electrophoresis on 1.5% agarose gel, PCR bands were visualized by ultraviolet illumination.
ASCs expressed mesenchymal stem cell markers CD29 and CD44, and were negative for hematopoietic markers CD31 and CD34 (Fig. 1). ASCs treated with adipogenic medium developed intracellular lipid-containing droplets that accumulated the lipid dye Oil red O (Fig. 2A and 2B). ASCs cultured in osteogenic medium formed aggregates, and stained positively for alkaline phosphatase (Fig. 2C, D). Calcium deposits were observed in Alizarin red S staining (Fig. 2E).
Treatment with 50% ASC-CM for 4 days significantly increased the proliferation of HaCaT cells and dermal fibroblasts compared with the vehicle-treated control (p<0.05) (Fig. 3). The proliferation of HaCaT cells was enhanced more than that of dermal fibroblasts by ASC-CM (218.8±17.3% vs. 126.9±3.3%).
In the in vitro wound healing assay, 50% ASC-CM significantly enhanced the wound healing by 2.43-fold over control level after 24-hour incubation (130.91±33.46 µm vs. 53.81±6.23 µm, p<0.05) (Fig. 4). Treatment with 50% ASC-CM for 48 hours also promoted wound healing by the same degree (267.73±42.56 µm vs. 110.25±12.35 µm, p<0.05).
To evaluate the effect of ASC-CM on the ability of dermal fibroblasts to contract collagen lattices, 50% ASC-CM was added to the floating fibroblast-populated collagen lattice and the size of the collagen lattice was measured after 5 days. ASC-CM significantly reduced the size of collagen lattice compared with vehicle-treated control (6.22±0.51 cm2 vs. 7.73±0.10 cm2, p<0.05) (Fig. 5).
Treatment with 50% ASC-CM for 6 hours significantly increased the mRNA expression of the type I procollagen α1 chain gene in dermal fibroblasts by 1.27-fold over vehicle-treated control (p<0.05) (Fig. 6).
ASCs are multipotent cells that can differentiate along multiple cell lineages, including adipogenic, osteogenic, and chondrogenic pathways7,8. The cultured ASCs of this study could differentiate into adipogenic and osteogenic lineages in specific culture medium. ASCs showed the same immunophenotypes as previously described7,11. They expressed the mesenchymal stem cell markers CD29 and CD44, but not the hematopoietic markers CD31 and CD34.
Cutaneous wound healing is a dynamic, interactive process involving the interaction of cells in the dermis and epidermis, including fibroblasts and keratinocytes, as well as the release of chemical mediators from inflammatory cells. Wound healing proceeds in three overlapping phases: inflammation, tissue formation, and tissue remodeling12. Keratinocytes, fibroblasts, and endothelial cells play essential roles in the wound healing process including re-epithelialization, granulation tissue formation, and angiogenesis.
ASCs combined with atelocollagen matrix improve wound healing in healing-impaired db/db mice9, and a conditioned medium of ASCs alone accelerates wound healing in mice13. These results indicate that the paracrine effects of ASCs have an important role in their stimulatory effect on wound healing. ASCs have a stimulatory effect on endothelial cells in a paracrine fashion. Proangiogenic factors such as vascular endothelial growth factor (VEGF) are secreted at a high level from ASCs, and ASC-CM accelerates the proliferation of endothelial cells11,13. This paracrine effect of ASCs is thought be one of the proangiogenic mechanisms of ASCs in vivo11. In the case of keratinocytes and dermal fibroblasts, the paracrine effects of ASCs have not been well demonstrated. In limited studies, conditioned media of ASCs promoted the proliferation, migration, and production of extracellular matrix components of dermal fibroblasts13,14.
During re-epithelialization, a wedge-shaped mass of keratinocytes moves across the granulation tissue. At the leading edge of the wedge are migrating keratinocytes, which leave a stratified layer of proliferating keratinocytes. The keratinocytes that participate in these processes of migration and proliferation are derived from at least two anatomic locations including those cells in close proximity to the wound and epidermal cells of nearby hair bulges15. Keratinocytes are stimulated to migrate and proliferate by a number of factors during re-epithelialization. Among growth factors and cytokines, epidermal growth factor (EGF) and transforming growth factor (TGF)-β play a role in keratinocyte migration16,17. In addition, keratinocyte proliferation is stimulated by EGF, basic fibroblast growth factor (bFGF) and insulin-like growth factor (IGF)-117,18. Conditioned media of BMSCs promote the proliferation and migration of mouse keratinocytes6, but the effects of ASC-CM on keratinocytes have not been demonstrated. Our study revealed that ASC-CM enhances the proliferation of HaCaT cells (i.e., immortalized human keratinocytes) and accelerates in vitro wound healing of HaCaT cells.
Fibroblasts are the most important mesenchymal cells involved in wound healing. Fibroblasts in the dermis around a wound proliferate and migrate into the wound space. After migrating into the wound, they commence the synthesis of extracellular matrix components, including types I and III collagen, and participate in the formation of granulation tissue15. Platelet-derived growth factor (PDGF), TGF-β, nerve growth factor, and connective tissue growth factor (CTGF) act as chemoattractants for fibroblasts19. EGF, FGF, PDGF, TGF-β, CTGF, and IGF-1 promote the proliferation of fibroblasts, and the collagen production of fibroblasts is also stimulated by a number of growth factors, including FGF-2, PDGF, TGF-β, CTGF, and IGF-119,20. In this study, ASC-CM increased the proliferation and transcription of type I procollagen α1 chain gene of fibroblasts. These results are similar to a previous report14, and ASC-CM harvested under hypoxia promotes collagen synthesis compared with that harvested under normoxia13.
During the tissue remodeling phase of wound healing, the wound begins to contract. Fibroblasts assume a myofibroblast phenotype characterized by large bundles of actin-containing microfilaments disposed along the cytoplasmic face of the plasma membrane of the cells12. The appearance of the myofibroblasts corresponds to the commencement of contraction of the wound. TGF-β1 or β2 and PDGF stimulate the phenotype change of fibroblasts to myofibroblasts and wound contraction21,22. The effects of ASC-CM on wound contraction or contractility of fibroblasts have not been reported. In this study, ASC-CM increased the contraction of fibroblast-populated collagen lattice, suggesting that ASC-CM induce the phenotype change of fibroblasts to myofibroblasts.
ASCs produce various growth factors including PDGF, IGF, keratinocyte growth factor, bFGF, TGF-β, hepatocyte growth factor, and VEGF14,23, and hypoxic conditions stimulate the production of some of these13. The effects of ASC-CM on HaCaT cells and fibroblasts may largely depend on the complex action of these cytokines.
In conclusion, our data demonstrate that ASCs enhance the functions of keratinocytes and dermal fibroblasts in a paracrine fashion. The stimulatory effect of ASCs on cutaneous wound healing may be partially mediated by the paracrine effects of ASCs on other skin cells. Application of ASCs or ASCs-derived molecules could be an innovative therapeutic approach in the treatment of chronic wounds and other conditions.
Figures and Tables
 | Fig. 1Adipose-derived stem cells at passage 4 expressed CD29 and CD44, whereas hematopoietic cell marker CD31 and CD34 were not expressed (immunoperoxidase, ×100). |
 | Fig. 2Adipose-derived stem cell (ASCs) could differentiate toward the adipogenic and osteogenic lineages. ASCs at passage 4 were treated with adipogenic medium (A, B) or osteogenic medium (C~E) for 3 weeks. Oil red O staining (B), alkaline phosphatase staining (D), and Alizarin red S staining (E) were performed to confirm adipo- and osteogenesis. |
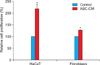 | Fig. 3Proliferation of HaCaT cells and dermal fibroblasts was increased by the conditioned medium of adipose-derived stem cell (ASC-CM) in 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. HaCaT cells and dermal fibroblasts were treated with 50% ASC-CM or Dulbecco's modified Eagle's medium (control) for 4 days. The values shown are the mean±standard error of the mean of six independent experiments performed in triplicate wells. *p<0.05 vs. the control group. |
 | Fig. 4The conditioned medium of adipose-derived stem cell (ASC-CM) promote in vitro wound healing of HaCaT cells. (A) Monolayers of confluent HaCaT cells were wounded with a micropipette tip, and then treated with 50% ASC-CM or Dulbecco's modified Eagle's medium (control). Photographs were taken at 24 hours and 48 hours. (B) The width of healed area was calculated with an image analysis program. The values shown are the mean±standard error of the mean of six independent experiments. *p<0.05 vs. the control group. |
 | Fig. 5The conditioned medium of adipose-derived stem cell (ASC-CM) increased the contraction of fibroblast-populated collagen lattice. (A) Collagen lattice was treated with 50% ASC-CM or Dulbecco's modified Eagle's medium (control) for 5 days. (B) The size of collagen lattice was measured with an image analysis program. The values shown are the mean±standard error of the mean of six independent experiments. *p<0.05 vs. the control group. |
 | Fig. 6Transcription of type I procollagen α1 chain gene is up-regulated in fibroblasts by the conditioned medium of adipose-derived stem cell (ASC-CM). (A) Fibroblasts were treated with 50% ASC-CM or Dulbecco's modified Eagle's medium (control) for 6 hours and then reverse transcription-polymerase chain reaction was performed. (B) The values of densitometric quantification are the mean±standard error of the mean of six independent experiments. *p<0.05 vs. the control group. |
ACKNOWLEDGMENT
This study was supported by Grant No. 04-2006-1050 from the Seoul National University Hospital Research Fund.
References
1. Steeper R. A critical review of the aetiology of diabetic neuropathic ulcers. J Wound Care. 2005. 14:101–103.

2. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.

3. Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001. 282:148–152.

4. Fuchs S, Baffour R, Zhou YF, Shou M, Pierre A, Tio FO, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001. 37:1726–1732.

5. Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007. 25:2648–2659.

6. Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008. 3:e1886.

7. Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002. 13:4279–4295.

8. Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006. 99:1285–1297.

9. Nambu M, Kishimoto S, Nakamura S, Mizuno H, Yanagibayashi S, Yamamoto N, et al. Accelerated wound healing in healing-impaired db/db mice by autologous adipose tissue-derived stromal cells combined with atelocollagen matrix. Ann Plast Surg. 2009. 62:317–321.

10. Park JS, Park WY, Cho KA, Kim DI, Jhun BH, Kim SR, et al. Down-regulation of amphiphysin-1 is responsible for reduced receptor-mediated endocytosis in the senescent cells. FASEB J. 2001. 15:1625–1627.

11. Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, et al. Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem. 2006. 17:279–290.

13. Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009. 17:540–547.

14. Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007. 48:15–24.

15. Baum CL, Arpey CJ. Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg. 2005. 31:674–686.

16. Sarret Y, Woodley DT, Grigsby K, Wynn K, O'Keefe EJ. Human keratinocyte locomotion: the effect of selected cytokines. J Invest Dermatol. 1992. 98:12–16.

17. Bhora FY, Dunkin BJ, Batzri S, Aly HM, Bass BL, Sidawy AN, et al. Effect of growth factors on cell proliferation and epithelialization in human skin. J Surg Res. 1995. 59:236–244.

18. Ishimoto S, Ishibashi T, Bottaro DP, Kaga K. Direct application of keratinocyte growth factor, basic fibroblast growth factor and transforming growth factor-alpha during healing of tympanic membrane perforation in glucocorticoid-treated rats. Acta Otolaryngol. 2002. 122:468–473.

19. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003. 83:835–870.

20. Yamaguchi Y, Crane S, Zhou L, Ochoa SM, Falanga V. Lack of co-ordinate expression of the alpha1(I) and alpha1(III) procollagen genes in fibroblast clonal cultures. Br J Dermatol. 2000. 143:1149–1153.

21. Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA. 1988. 85:4894–4897.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download