Abstract
Chronic inflammatory skin diseases such as atopic dermatitis, psoriasis or rosacea are very common. Although their exact pathogenesis is not completely understood all three diseases are characterized by dysregulation of cutaneous innate immunity. Cathelicidin LL-37 is an important effector molecule of innate immunity in the skin and atopic dermatitis, psoriasis or rosacea show defects in cathelicidin expression, function or processing. In atopic dermatitis, cathelicidin induction might be disturbed resulting in defective antimicrobial barrier function. In contrast, psoriasis is characterized by overexpression of cathelicidin. However to date it is unclear whether pro- or anti-inflammatory functions of cathelicidin predominate in lesional skin in psoriasis. In rosacea, cathelicidin processing is disturbed resulting in peptide fragments causing inflammation, erythema and telangiectasias. In this review, the current evidence on the role of cathelicidin LL-37 in the pathogenesis of inflammatory skin diseases will be outlined. As cathelicidin LL-37 might also serve as a future treatment target potential novel treatment strategies for those diseases will be discussed.
Our skin is the first barrier against the outside environment. In order to provide an efficient defense the skin is equipped with various innate mechanisms against invading microbial pathogens. Most do not require the specific recognition of the invading pathogen and within cutaneous innate immunity three distinct barriers can be identified: a chemical, a physical and a cellular barrier. While a low pH and small cationic peptides with antimicrobial activity contribute to the chemical shield on the surface of the skin, the stratum corneum forms the initial physical barrier. Epidermal keratinocytes form the first cellular barrier against infectious agents1. These cells together with professional antigen presenting cells such as dendritic cells and dermal macrophages form the first line of cellular innate immunity in the skin. All these cells are equipped with sensors and communicate with each other upon microbial challenge or a danger signal. Subsequently, further immune or pro-inflammatory cascades are triggered providing an adequate and coordinated immune response.
Among the soluble factors secreted onto the cutaneous surface AMPs play a particularly important role in innate immunity. AMPs are evolutionary well-conserved gene-encoded, short (<100 amino acids), amphipathic molecules with hydrophobic and cationic amino acids arranged spatially2. AMPs form predominantly two different secondary structures, disulfide-rich peptides form beta-sheets while linear peptides form alpha-helices3. Their amphipathic structure allows those peptides to be soluble in aqueous environments but also to interact with lipid membranes3. Initially, AMPs were identified as endogenous antibiotics due to their potential to kill various pathogens by disrupting their membranes. They have broad spectrum antimicrobial activity and are able to kill gram-positive and gram-negative bacteria, viruses and fungi4. Keratinocytes and other resident cells in the skin such as eccrine gland cells, mast cells and sebocytes produce and secrete AMPs. In addition, invading immune cells (e.g. neutrophils, natural killer cells) contribute to the pool of AMPs in the skin4-8.
Resident and infiltrating cells synthesize an impressive array of AMPs and to date several hundred of different peptides with antimicrobial function have been identified in human skin9-11. Interestingly, many AMPs were first characterized for other biological activities and their antimicrobial function was later identified. As an example the leukocyte protease inhibitor - also known as α-melanocyte stimulating hormone - exerts antimicrobial activity when tested in culture9.
Importantly, the production of AMPs in the skin is a dynamic defense mechanism: While some AMPs are expressed constitutively in the skin, the production of others is highly increased in danger situations such as skin injury or infections12-15. Furthermore, the expression and function of AMPs are regulated on the transcriptional and post-transcriptional level. Most AMPs are synthesized as pro-peptides and activated after proteolytic cleavage from their precursor molecules16,17. Consequently, induction of transcription and increased processing are needed to provide the skin with enhanced AMP activity.
Two important and well-studied AMP families in human skin are the defensins and the cathelicidins18,19. β-defensin (HBD) 2 was the first skin-derived AMP characterized in man. HBD2 is most effective against gram-negative bacteria whereas HBD3 from the same AMP family has a broader spectrum of antimicrobial action13,20. HBD2 is induced in skin inflammation and infection21 and Gläser et al.22 recently showed that HBD2 and 3 are inducible by ultraviolet-B (UVB) irradiation as well23. In contrast, HBD1 is constitutively expressed in human skin.
While there are several gene-encoded defensins, e.g. α- and β-defensins, to date only one single cathelicidin gene has been identified in man and designated cathelicidin antimicrobial peptide (CAMP)24,25. Like many AMP genes CAMP encodes for a pro-peptide which is composed of an N-terminal cathelin domain and a C-terminal peptide with antimicrobial activity26. The first active cathelicidin identified was LL-37 - a 37 amino acid long peptide with broad antimicrobial activity. LL-37 forms an α-helix in aqueous solution which enables the peptide to disrupt both bacterial membranes and viral envelopes9. Even anti-fungal activity of LL-37 in Candida infections was reported27-29. On the skin surface, active cathelicidin peptides such as LL-37 are cleaved from the inactive precursor by serine proteases of the kallikrein family16,19,30.
As LL-37 gains its antimicrobial activity after cleavage from the pro-form processing of cathelicidin by skin proteases is an important regulatory mechanism of cathelicidin mediated antimicrobial activity. In skin, LL-37 is synthesized by epithelial cells but also provided by infiltrating immune cells such as neutrophils which transport LL-37 to infected or wounded skin. In healthy skin, cathelicidin expression is barely detected in keratinocytes. In contrast, during infection or injury cathelicidin production is strongly induced in these cells14.
Apart from the antimicrobial activity LL-37 has additional functions in the activation and the control of immune responses: LL-37 increases cytokine and chemokine liberation from local cells and leucocytes and has chemotactic effects on a large number of immune cells4. In addition, chemokine and cytokine release is induced by LL-37 in mast cells or keratinocytes31. In cooperation with the cytokines LL-37 enhances innate immune responses by multiple pathways4,32. Furthermore, LL-37 enhances the proliferation of endothelial cells and influences angiogenesis33. These attributes complement the antimicrobial functions of LL-37 and have lead to the perception of LL-37 as not only an antimicrobial but an "alarmin"-peptide34.
On a molecular level LL-37 mediates its "alarmin" functions on immune or resident cells in a ligand-receptor mediated or a receptor-independent manner resulting in increased host responses35. In doing so, LL-37 influences adenosine triphosphate-receptor P2X7 and Toll-like receptor (TLR) signaling in immune cells, epidermal growth factor receptor transactivation or intracellular Ca2+ mobilization31,36-38.
The dual role of cathelicidin - the antimicrobial and the alarmin function - suggests a central role for this peptide in cutaneous innate immunity. Consequently, dysfunction of the "alarmin"-function of cathelicidin LL-37 could play a role of in the pathogenesis of inflammatory skin disease to the same extent as impaired antimicrobial activity.
Emerging evidence suggests that indeed a number of inflammatory skin diseases are characterized by dysregulated expression or function of cathelicidin peptides. In this review, the current knowledge on the role of cathelicidin in the pathogenesis of atopic dermatitis (AD), rosacea, psoriasis and hidradenitis suppurativa (HS) will be presented and discussed.
AD is a very common inflammatory skin disease with a chronic course. Patients suffering from AD show an increased susceptibility to infections by viruses, bacteria or fungi and have an altered skin microflora39. A primary defense barrier defect caused by structural defects (e.g. mutations in the filaggrin gene) or by an immunoglobulin E (IgE)-mediated immunonologic disorder with IgE-mediated allergic sensitization and an epithelial-barrier dysfunction as a consequence of the local inflammation are discussed39.
A deficient innate antimicrobial barrier in AD patients was first proposed when an impaired expression of AMPs such as cathelicidin and defensins was detected in lesional skin in AD40. In particular, induction of cathelicidin mRNA transcription in response to wounding is suppressed in AD lesions as compared to healthy skin41. This could be explained by the altered tissue microenvironment in AD skin: Th2 cytokines such as interleukin (IL)-4 and IL-, which are highly elevated in AD skin, suppress cathelicidin induction in keratinocytes42. As itching is a hallmark of AD and scratching results in skin wounding failure to upregulate cathelicidin in response to injury could decrease the cutaneous antimicrobial activity in AD skin41.
Furthermore, it has been hypothesized that the increased rate of microbial superinfections in AD may be caused by reduced AMP expression as a consequence of immunosuppressive therapy. In this context, topically applied corticosteroids and the calcineurin inhibitor pimecrolimus reduce the expression of several AMPs in skin in AD compared with healthy controls43.
In contrast, other groups found enhanced expression of cathelicidin LL-37 mRNA and protein expression in lesional skin compared with non-lesional skin in AD44. Also, non-lesional skin of atopic and non-atopic children shows no significant difference in cathelicidin LL-37 and HBD-2 expression45. Also, the serum levels of cathelicidin LL-37 in AD children did not differ from healthy controls and increased in subgroups with more severe disease46. Finally, serum LL-37 did not differ between patients with or without relevant bacterial superinfection.
Nevertheless, two clinical studies identified subgroups of AD patients with severe infectious complications and a history of dermatitis herpeticatum in the past, which showed defective upregulation of AMPs47,48.
Hence, the role of cathelicidin LL-37 in the pathogenesis of AD is still unclear. Further studies - in particular in subgroups of AD patients suffering from severe AD with infectious complications - are needed to exactly characterize the role of cathelicidin LL-37 in AD.
Rosacea is another inflammatory skin disease mainly affecting the central portions of the face. The disease often affects fair-skinned individuals and shows a chronic relapsing course. Rosacea occurs mainly in adults around the age of 30 years and typically predominates in females49. The clinical presentations of rosacea include erythema and telangiectasias, pustules and erythematous papules, rarely nodules or edema. The pathophysiology of rosacea is incompletely understood but involves a complex interaction of different factors and pathways leading to a chronic inflammatory and vascular response.
As cathelicidin LL-37 has pro-inflammatory "alarmin" functions and affects vascular growth the expression of cathelicidin was investigated in rosacea recently. Indeed, cathelicidin is strongly increased lesional skin in rosacea compared to the skin of non-affected individuals50.
As mentioned above, processing of the precursor molecule is a crucial step in activating the different functions of cathelicidin peptides. In the skin cathelicidin is processed by serine proteases of the kallikrein family (particularly kallikrein 5 [stratum corneum tryptic enzyme] and kallikrein 7 [stratum corneum chymotryptic enzyme])16. The main resulting peptide is LL-37 - however, LL-37 can be processed further to smaller peptide fragments. Also these smaller peptide fragments exert immune functions but differ in their antimicrobial and immune activating capacities51.
In lesional skin in rosacea the cutaneous protease activity is enhanced and increased expression of serine proteases such as kallikrein 5 can be observed50. Furthermore, variant cathelicidin peptides and smaller fragments can be detected. Thus, in rosacea increased levels of the vasoactive and inflammatory host-defense peptide LL-37 and its proteolytic peptide fragments are found which can be explained by an abnormal cathelicidin production and pathologic protease activity50,52.
Confirming a pathogenic role of cathelicidin in rosacea, injection of these peptide fragments found in skin of rosacea patients into the skin of mice leads to a rosacea-like disease50. In contrast, the isolated increase of protease activity in cathelicidin knock-out mice does not cause dermal inflammation50.
However, the mechanisms underlying the increased cathelicidin production and the enhanced protease activity in skin of rosacea patients are to date unknown. Both seem to be regulated by different signaling pathways with retinoid-, vitamin D- and cytokine-activated cascades playing important roles53.
Cathelicidin LL-37 expression in human keratinocytes is regulated by the vitamin D pathway54. This could explain why rosacea occurs mainly in the face as exposition to ultraviolet (UV) light triggers activation of vitamin D in keratinocytes and subsequent cathelicidin expression54,55.
Recently, a second - vitamin D independent - pathway triggering the induction of cathelicidin synthesis in keratinocytes was identified: In keratinocytes, cathelicidin expression increases upon several external stimuli (such as infection, injuries, UV irradiation, and permeability barrier disruption) which also trigger endoplasmic reticulum (ER) stress. Indeed, ER stress increases CAMP expression via nuclear factor-κB-carbohydrateresponsive element binding proteinα activation independent of vitamin D receptor (VDR) activation demonstrating a novel role for ER stress in stimulating innate immunity56. This again could explain why rosacea patients often report on unspecific triggers (e.g. heat) which would mediate their pro-inflammatory activities through ER stress and cathelicidin induction.
But why is the cutaneous protease activity elevated in lesional skin in rosacea? As mentioned earlier resident cells in the skin such as keratinocytes express receptors sensing pathogen associated molecular patterns (PAMPs). TLRs are one family of PAMP sensors triggering further pro-inflammatory pathways. As an example, ligands for TLR2 are microbial structure molecules such as cell wall fragments but also chitin57,58. In involved skin in rosacea patients keratinocytes express elevated level of TLR257. Furthermore, TLR2 activation in keratinocytes leads to a higher expression of kallikrein proteases and higher protease activity (Fig. 1)57.
And what is the trigger for TLR2 in rosacea? Demodex mites are frequently found on the skin of rosacea patients and skin inflammation correlates with mite density59. Possibly, chitin released from these mites could serve as the trigger for TLR2 on keratinocytes and link demodex with increased protease activity and cathelicidin induced inflammation in rosacea (Fig. 1).
Together these observations possibly identify a complex pro-inflammatory cascade involving demodex, TLRs, cathelicidin and proteases in the pathogenesis of rosacea. Nevertheless, several of these mechanisms could be exploited for novel therapies to be discussed in the following paragraphs.
Psoriasis is a third inflammatory skin disease associated with abnormal expression and activity of AMPs60. An autoinflammatory reaction is suspected to play a major role in the course of the disease however the triggers of inflammation in psoriasis remain unknown.
Cathelicidin LL-37 is strongly increased in skin in psoriatic plaques and recently LL-37 was identified as a critical factor for the activation of an auto-inflammatory cascade in psoriasis61. LL-37 isolated from lesional psoriatic skin forms complexes with human self-DNA. These LL-37/self-DNA complexes are sensed by dermal plasmacytoid dendritic cells (pDCs) through TLR961. In turn, activated pDCs secrete large amounts of interferon α to activate further T-cell mediated immune responses. Thus, LL-37 converts inert self-DNA into a potent trigger of interferon production by pDCs in psoriatic skin61.
Subsequently, the capacity of LL-37 to bind to self-DNA and influence inflammation prompted further studies in psoriasis patients. DNA is not only sensed by TLRs but also by absent in melanoma 2 (AIM2), a cytosolic DNA receptor which upon activation triggers inflammasome activation and IL-1β secretion62. AIM2 expression and IL-1β secretion is high in psoriatic lesions but not in healthy skin62. Furthermore, abundant cytosolic DNA was detected in keratinocytes in psoriatic lesions, which triggered the release of IL-1β via the AIM2 inflammasome62. Again, LL-37 bound cytosolic DNA but this time neutralized its pro-inflammatory activity: Cytosolic DNA complexed with cathelicidin LL-37 did not activate the AIM2 inflammasome and subsequent IL-1β secretion was inhibited.
Thus, these studies revealed a contrasting role of cathelicidin in self-DNA mediated inflammation in lesional skin in psoriasis: While dermal LL-37 binds self-DNA and mediates activation of pDCs, epidermal LL-37 complexes with cytosolic DNA in keratinocytes and blocks inflammasome activation and IL-1β release. To date it is unclear which effect - the pro- or the anti-inflammatory - of cathelicidin LL-37 is predominating. Studies in patients under treatment could contribute to the understanding of the role of LL-37 in psoriasis. These studies will be discussed in more detail in the following paragraphs.
HS is a chronic inflammatory skin disease often resulting in exzessive scarring and fistulation. The axillar, gluteal, inguinal and genital skin areas are often affected. As the pathophysiology of HS is unclear, medical treatment is difficult or even frustrating. To date, surgical excision of affected skin areas is the treatment of choice. AMPs are strongly expression and secreted by the apocrine sweat glands, distal hair follicle epithelium, and epidermis in HS63. In particular, immunoreactivity of the cathelicidin LL-37 was increased in HS lesions. The pro-inflammatory functions of LL-37 could trigger local disease excacerbation and thus promote HS development. However, the triggers and pathways of AMP regulated innate immunity in HS are for the most part unclear and require further research work.
The increasing data on the role of cathelicidin and other AMPs in inflammatory skin diseases has prompted the idea that through targeting AMP expression cutaneous inflammation might be ameliorated. In order to influence AMP expression and function the detailed knowledge of the mechanisms and pathways regulation AMP expression in skin is a prerequisite. As mentioned earlier some regulatory pathways have been identified during the past years: As an example, vitamin D directly activates cathelicidin gene CAMP transcription and LL-37 peptide expression in several cell types such as keratinocytes and monocytes64,65. Importantly, increased cathelicidin expression is paralleled by increased cathelicidin peptide activity34. Restoring antimicrobial activity or balancing "alarming" activities of AMPs could be novel goals of topical or systemic treatments for inflammatory skin diseases66.
The rate of atopy and in particular the prevalence of AD is very high in industrialized countries. As patients with AD show structural defects in their cutaneous barrier and dysfunction of cutaneous innate immunity leading to microbial superinfections restoration of the antimicrobial defense shield could be beneficial. Vitamin D directly regulates cathelicidin AMP expression in keratinocytes and a link between systemic vitamin D serum levels, sun exposure and atopy has been discussed67.
Indeed, oral supplementation of vitamin D induces cathelicidin production in skin in AD patients68. Also, systemic treatment with a VDR agonist leads to healing of experimental allergen-triggered eczema possibly through effects on the cutaneous barrier69. Topical vitamin D application on human skin also increases cathelicidin immunoreactivity14. Still, to date it is unclear whether AD patients treated with vitamin D show a decreased rate of infectious complications as a consequence of increased cathelicidin expression.
As mentioned earlier, human skin is able to synthesize active vitamin D from precursor molecules under the influence of UV irradiation. Even in a culture dish UV-B irradiation of human keratinocytes supplemented with 7-dehydrocholesterol triggers the synthesis of hormonally active calcitriol, which then differentially affects expression of AMPs cathelicidin and HBD255. In a clinical setting, UVB phototherapy increases the endogenous production of vitamin D in AD patients which is accompanied by healing of eczema lesions70. These effects might be mediated by improved vitamin D balance, the local cytokine network and/or AMP expression70. However, larger, prospective, multi-center studies are needed to clarify the role AMP regulated barrier functions in AD.
The pathways involved in increased and dysfunctional cathelicidin in the skin of patients with rosacea are complex. However, due to the multiple regulatory steps involved several strategies are possible for topical and systemic therapies (Fig. 1).
As an example, chitin released from demodex mites possibly triggers TLR2 activation and subsequent protease activity in the skin of rosacea patients. Thus, decreasing the load of demodex mites on the skin or blocking of TLR2 could decrease protease activity and cutaneous inflammation. Indeed, inhibition of demodex has been suggested in the treatment of rosacea before and decreased mite density associates with reduced inflammation in clinical studies71. Retinoids, which are commonly used in the treatment of rosacea, block TLR2 activity and could mediate their effects through this mechanism as well (Fig. 1).
The increased protease activity is central to the pro-inflammatory effects of dysfunctional cathelicidin peptides in rosacea50. Hence, topical or systemic therapies inhibiting pathologic protease activity could exert anti-inflammatory effects in rosacea. Indeed, some established and clinically effective therapies probably exert their beneficial effect through this mechanism. As an example, tetracyclines, such as doxycycline, can inhibit proteases and ameliorate cutaneous inflammation in rosacea34,72,73.
Importantly, these anti-inflammatory effects are independent of the antimicrobial effect of e.g. doxycycline and the doses needed for clinical effect are probably lower than those needed to treat infections (Fig. 1).
Azelaic acid - another topical drug commonly used for rosacea74 - might reduce the protease activity in keratinocytes, too75.
Another way to interfere with the outlined pro-inflammatory cascade could be the blockade of increased cathelicidin production in lesional skin in rosacea. As mentioned earlier, vitamin D controls the expression of cathelicidin and UVB triggers activation of vitamin D from precursor molecules65. Thus, the advice to rosacea patients to avoid exposure to sun light might find its scientific basis in those observations.
Also, alterations VDR gene have been described in patients with severe rosacea (rosacea conglobata) suggesting that vitamin D signaling is indeed affecting the pathogenesis of rosacea76. Drugs which interfere with vitamin D signaling and therefore are able to control the expression and processing of cathelicidins might offer a new approach in the management of rosacea77.
As cathelicidin might be a central factor in the pathogenesis of psoriasis therapies targeting cathelicidin expression might influence the course of the disease. On the one hand, the blockage of dermal LL-37 or a decrease in dermal cathelicidin deposition might interrupt the vicious circle of cutaneous inflammation induced by elevated levels of LL-37/self-DNA-complexes which leads to pDC activation61,78. On the other hand, increased epidermal cathelicidin could lead to sustained AIM2 inflammasome inhibition and a decrease in cutaneous inflammasome activity.
Expression of cathelicidin in keratinocytes is regulated by vitamin D. Moreover, vitamin D analogs have been used in the treatment of psoriasis for a long time. However, the molecular mechanisms behind their clinical effects were not completely lucid. vitamin D analogs bind to the VDR followed by binding of the VDR binds to vitamin D responsive element in the promoter region of the cathelicidin gene34,66.
Topical treatment with vitamin D analogs, such as calcipotriol, decrease inflammation and reduce morphological changes in psoriatic lesions79. At the same time calcipotriol treatment decreases pro-inflammatory cytokines but strongly increases the expression of cathelicidin66.
Similar to AD, UVB phototherapy is also used for the treatment of affected skin in psoriasis vulgaris. Repeated treatments of psoriasis patients with narrowband UVB significantly decreased skin inflammation in psoriasis patients but at the same time strongly increased vitamin D serum levels and cutaneous cathelicidin expression70. Thus, established therapies targeting the vitamin D pathway reduce inflammation while increasing epidermal cathelicidin expression in psoriatic lesions. The anti-inflammatory effect of cathelicidin LL-37 on the AIM2 inflammasome pathway could be responsible for this effect.
Vitamin D serum levels can also be increased through oral supplementation and some studies have started to investigate if the vitamin D serum levels in patients with psoriasis correlate with cutaneous AMP expression. One study revealed that cathelicidin expression in lesional skin was higher in serum vitamin D sufficient groups compared to serum vitamin D deficient groups80. Apparently, vitamin D serum levels and cathelicidin expression were not sufficient to inhibit inflammation. Therefore, more prospective interventional studies testing, whether increasing vitamin D serum levels affects the course of psoriasis are needed.
Many inflammatory skin diseases such as AD, psoriasis and rosacea are characterized by dysregulated synthesis of AMPs. Cathelicidin LL-37 is one important AMP found in skin. As cathelicidin exerts antimicrobial but also immune functions dysregulated expression and processing of this AMP is involved in the pathogenesis of chronic inflammatory skin diseases. As the regulatory mechanisms of cathelicidin gene regulation and peptide processing become clearer strategies to influence these processes emerge. Furthermore, established therapies such as topical and systemic treatments for rosacea or psoriasis might mediate their effects through their impact on cathelicidin. Further research could identify novel targets and mechanisms, which could lead to innovative treatments for inflammatory skin diseases, through their effects on cathelicidin.
Figures and Tables
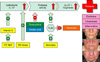 | Fig. 1The role of cathelicidin in the pathogenesis of rosacea and possible therapeutic implications. UV light increases the synthesis of vitamin D which induces cathelicidin expression in keratinocytes. ER stress is an alternative inducer of cathelicidin production. Increased protease activity in rosacea skin is possibly due to demodex mite colonization: Chitin released from mites triggers TLR2 receptor activation and increased protease activity. Subsequently, enhanced protease activity leads to increased cleavage of cathelicidin LL-37 and further fragments. These fragments trigger inflammation, erythema and telangiectasias. Doxycycline, azelaic acid and retinoids mediate their beneficial effects in rosacea possibly by interfering with this pro-inflammatory system through different mechanisms. UV: ultraviolet, ER: endoplasmic reticulum, TLR: Toll-like receptor. |
ACKNOWLEDGMENT
This study was funded by the Deutsche Forschungsgemeinschaft (Emmy Noether Program SCHA 979/3-1 to J.S.) and the Fritz Thyssen Stiftung.
References
1. Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009. 9:679–691.

2. Pasupuleti M, Schmidtchen A, Malmsten M. Antimicrobial peptides: key components of the innate immune system. Crit Rev Biotechnol. 2011. [Epub ahead of print].

3. Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003. 55:27–55.

4. Schauber J, Gallo RL. Expanding the roles of antimicrobial peptides in skin: alarming and arming keratinocytes. J Invest Dermatol. 2007. 127:510–512.

5. Braff MH, Zaiou M, Fierer J, Nizet V, Gallo RL. Keratinocyte production of cathelicidin provides direct activity against bacterial skin pathogens. Infect Immun. 2005. 73:6771–6781.

6. Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J Invest Dermatol. 2002. 119:1090–1095.

7. Dorschner RA, Pestonjamasp VK, Tamakuwala S, Ohtake T, Rudisill J, Nizet V, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001. 117:91–97.

8. Antal AS, Dombrowski Y, Koglin S, Ruzicka T, Schauber J. Impact of vitamin D3 on cutaneous immunity and antimicrobial peptide expression. Dermatoendocrinol. 2011. 3:18–22.

9. Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006. 306:91–110.

10. Zhang G, Ross CR, Blecha F. Porcine antimicrobial peptides: new prospects for ancient molecules of host defense. Vet Res. 2000. 31:277–296.

11. Wang Z, Wang G. APD: the antimicrobial peptide database. Nucleic Acids Res. 2004. 32(Database issue):D590–D592.

12. Peric M, Koglin S, Kim SM, Morizane S, Besch R, Prinz JC, et al. IL-17A enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J Immunol. 2008. 181:8504–8512.

13. Harder J, Bartels J, Christophers E, Schroder JM. Isolation and characterization of human beta -defensin-3, a novel human inducible peptide antibiotic. J Biol Chem. 2001. 276:5707–5713.

14. Schauber J, Dorschner RA, Coda AB, Büchau AS, Liu PT, Kiken D, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007. 117:803–811. Epub 2007 Feb 8.

15. Wolk K, Witte E, Wallace E, Döcke WD, Kunz S, Asadullah K, et al. L-22 regulates the expression of genes responsible for antimicrobial defense, cellular differentiation, and mobility in keratinocytes: a potential role in psoriasis. Eur J Immunol. 2006. 36:1309–1323.

16. Yamasaki K, Schauber J, Coda A, Lin H, Dorschner RA, Schechter NM, et al. Kallikrein-mediated proteolysis regulates the antimicrobial effects of cathelicidins in skin. FASEB J. 2006. 20:2068–2080.

17. Michaelson D, Rayner J, Couto M, Ganz T. Cationic defensins arise from charge-neutralized propeptides: a mechanism for avoiding leukocyte autocytotoxicity? J Leukoc Biol. 1992. 51:634–639.

18. Selsted ME, Ouellette AJ. Mammalian defensins in the antimicrobial immune response. Nat Immunol. 2005. 6:551–557.

19. Zaiou M, Gallo RL. Cathelicidins, essential gene-encoded mammalian antibiotics. J Mol Med (Berl). 2002. 80:549–561.

20. Harder J, Siebert R, Zhang Y, Matthiesen P, Christophers E, Schlegelberger B, et al. Mapping of the gene encoding human beta-defensin-2 (DEFB2) to chromosome region 8p22-p23.1. Genomics. 1997. 46:472–475.

22. Gläser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schröder JM, et al. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J Allergy Clin Immunol. 2009. 123:1117–1123.

23. Zhao C, Wang I, Lehrer RI. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996. 396:319–322.

24. Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995. 368:173–176.

25. Larrick JW, Hirata M, Zhong J, Wright SC. Anti-microbial activity of human CAP18 peptides. Immunotechnology. 1995. 1:65–72.

26. Gudmundsson GH, Agerberth B, Odeberg J, Bergman T, Olsson B, Salcedo R. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996. 238:325–332.

27. López-García B, Lee PH, Yamasaki K, Gallo RL. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005. 125:108–115.

28. López-García B, Lee PH, Gallo RL. Expression and potential function of cathelicidin antimicrobial peptides in dermatophytosis and tinea versicolor. J Antimicrob Chemother. 2006. 57:877–882.

29. Tsai PW, Yang CY, Chang HT, Lan CY. Human antimicrobial peptide LL-37 inhibits adhesion of Candida albicans by interacting with yeast cell-wall carbohydrates. PLoS One. 2011. 6:e17755.

30. Braff MH, Hawkins MA, Di Nardo A, Lopez-Garcia B, Howell MD, Wong C, et al. Structure-function relationships among human cathelicidin peptides: dissociation of antimicrobial properties from host immunostimulatory activities. J Immunol. 2005. 174:4271–4278.

31. Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, et al. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007. 157:1124–1131.

32. Yu J, Mookherjee N, Wee K, Bowdish DM, Pistolic J, Li Y, et al. Host defense peptide LL-37, in synergy with inflammatory mediator IL-1beta, augments immune responses by multiple pathways. J Immunol. 2007. 179:7684–7691.

33. Koczulla R, von Degenfeld G, Kupatt C, Krötz F, Zahler S, Gloe T, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003. 111:1665–1672.

34. Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008. 122:261–266.

35. Oppenheim JJ, Tewary P, de la Rosa G, Yang D. Alarmins initiate host defense. Adv Exp Med Biol. 2007. 601:185–194.

36. Tomasinsig L, Pizzirani C, Skerlavaj B, Pellegatti P, Gulinelli S, Tossi A, et al. The human cathelicidin LL-37 modulates the activities of the P2X7 receptor in a structure-dependent manner. J Biol Chem. 2008. 283:30471–30481.

37. Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, et al. Cathelicidin antimicrobial peptides block dendritic cell TLR4 activation and allergic contact sensitization. J Immunol. 2007. 178:1829–1834.

38. Tokumaru S, Sayama K, Shirakata Y, Komatsuzawa H, Ouhara K, Hanakawa Y, et al. Induction of keratinocyte migration via transactivation of the epidermal growth factor receptor by the antimicrobial peptide LL-37. J Immunol. 2005. 175:4662–4668.

40. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002. 347:1151–1160.

41. Mallbris L, Carlén L, Wei T, Heilborn J, Nilsson MF, Granath F, et al. Injury downregulates the expression of the human cathelicidin protein hCAP18/LL-37 in atopic dermatitis. Exp Dermatol. 2010. 19:442–449. Epub 2009 Jul 23.

42. Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006. 24:341–348.

43. Jensen JM, Ahrens K, Meingassner J, Scherer A, Bräutigam M, Stütz A, et al. Differential suppression of epidermal antimicrobial protein expression in atopic dermatitis and in EFAD mice by pimecrolimus compared to corticosteroids. Exp Dermatol. 2011. 20:783–788.

44. Ballardini N, Johansson C, Lilja G, Lindh M, Linde Y, Scheynius A, et al. Enhanced expression of the antimicrobial peptide LL-37 in lesional skin of adults with atopic eczema. Br J Dermatol. 2009. 161:40–47.

45. Goo J, Ji JH, Jeon H, Kim MJ, Jeon SY, Cho MY, et al. Expression of antimicrobial peptides such as LL-37 and hBD-2 in nonlesional skin of atopic individuals. Pediatr Dermatol. 2010. 27:341–348.

46. Leung TF, Ching KW, Kong AP, Wong GW, Chan JC, Hon KL. Circulating LL-37 is a biomarker for eczema severity in children. J Eur Acad Dermatol Venereol. 2012. 26:518–522.

47. Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006. 117:836–841.

48. Hata TR, Kotol P, Boguniewicz M, Taylor P, Paik A, Jackson M, et al. History of eczema herpeticum is associated with the inability to induce human β-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010. 163:659–661.

49. Chosidow O, Cribier B. Epidemiology of rosacea: updated data. Ann Dermatol Venereol. 2011. 138:Suppl 2. S124–S128.

50. Yamasaki K, Di Nardo A, Bardan A, Murakami M, Ohtake T, Coda A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007. 13:975–980.

51. Murakami M, Lopez-Garcia B, Braff M, Dorschner RA, Gallo RL. Postsecretory processing generates multiple cathelicidins for enhanced topical antimicrobial defense. J Immunol. 2004. 172:3070–3077.

52. Meyer-Hoffert U, Schröder JM. Epidermal proteases in the pathogenesis of rosacea. J Investig Dermatol Symp Proc. 2011. 15:16–23.

53. Morizane S, Yamasaki K, Kabigting FD, Gallo RL. Kallikrein expression and cathelicidin processing are independently controlled in keratinocytes by calcium, vitamin D(3), and retinoic acid. J Invest Dermatol. 2010. 130:1297–1306.

54. Schauber J, Gallo RL. The vitamin D pathway: a new target for control of the skin's immune response? Exp Dermatol. 2008. 17:633–639.

55. Peric M, Lehmann B, Vashina G, Dombrowski Y, Koglin S, Meurer M, et al. UV-B-triggered induction of vitamin D3 metabolism differentially affects antimicrobial peptide expression in keratinocytes. J Allergy Clin Immunol. 2010. 125:746–749.

56. Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, et al. Regulation of cathelicidin antimicrobial peptide expression by an endoplasmic reticulum (ER) stress signaling, vitamin D receptor-independent pathway. J Biol Chem. 2011. 286:34121–34130.

57. Yamasaki K, Kanada K, Macleod DT, Borkowski AW, Morizane S, Nakatsuji T, et al. TLR2 expression is increased in rosacea and stimulates enhanced serine protease production by keratinocytes. J Invest Dermatol. 2011. 131:688–697.

58. Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008. 181:4279–4286.

59. Georgala S, Katoulis AC, Kylafis GD, Koumantaki-Mathioudaki E, Georgala C, Aroni K. Increased density of Demodex folliculorum and evidence of delayed hypersensitivity reaction in subjects with papulopustular rosacea. J Eur Acad Dermatol Venereol. 2001. 15:441–444.

60. Hollox EJ, Huffmeier U, Zeeuwen PL, Palla R, Lascorz J, Rodijk-Olthuis D, et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet. 2008. 40:23–25.

61. Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007. 449:564–569.

62. Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Göss C, Anz D, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011. 3:82ra38.

63. Emelianov VU, Bechara FG, Gläser R, Langan EA, Taungjaruwinai WM, Schröder JM, et al. Immunohistological pointers to a possible role for excessive cathelicidin (LL-37) expression by apocrine sweat glands in the pathogenesis of hidradenitis suppurativa/acne inversa. Br J Dermatol. 2011. [Epub ahead of print].

64. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005. 19:1067–1077.

65. Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004. 173:2909–2912.

66. Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Büchau A, et al. Vitamin D analogs differentially control antimicrobial peptide/"alarmin" expression in psoriasis. PLoS One. 2009. 4:e6340.
67. Reinholz M, Ruzicka T, Schauber J. Vitamin D and its role in allergic disease. Clin Exp Allergy. 2011. [Epub ahead of print].

68. Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008. 122:829–831.

69. Hartmann B, Riedel R, Jörss K, Loddenkemper C, Steinmeyer A, Zügel U, et al. Vitamin D receptor activation improves allergen-triggered eczema in mice. J Invest Dermatol. 2012. 132:330–336.

70. Vähävihu K, Ala-Houhala M, Peric M, Karisola P, Kautiainen H, Hasan T, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010. 163:321–328.

71. Lazaridou E, Giannopoulou C, Fotiadou C, Vakirlis E, Trigoni A, Ioannides D. The potential role of microorganisms in the development of rosacea. J Dtsch Dermatol Ges. 2011. 9:21–25.

72. van Zuuren EJ, Gupta AK, Gover MD, Graber M, Hollis S. Systematic review of rosacea treatments. J Am Acad Dermatol. 2007. 56:107–115.

73. Del Rosso JQ, Webster GF, Jackson M, Rendon M, Rich P, Torok H, et al. Two randomized phase III clinical trials evaluating anti-inflammatory dose doxycycline (40-mg doxycycline, USP capsules) administered once daily for treatment of rosacea. J Am Acad Dermatol. 2007. 56:791–802.

74. Graupe K, Cunliffe WJ, Gollnick HP, Zaumseil RP. Efficacy and safety of topical azelaic acid (20 percent cream): an overview of results from European clinical trials and experimental reports. Cutis. 1996. 57:1 Suppl. 20–35.
75. Yamasaki K, Gallo R. Azelaic acid gel 15% alters kallikrein 5 and cathelicidin expression in epidermal keratinocytes, critical elements in the pathogenesis of rosacea. J Am Acad Dermatol. 2010. 60:AB1.
76. Jansen T, Krug S, Kind P, Plewig G, Messer G. BsmI polymorphism of the vitamin D receptor gene in patients with the fulminant course of rosacea conglobata (rosacea fulminans). J Dermatol. 2004. 31:244–246.

77. Segaert S. Vitamin D regulation of cathelicidin in the skin: toward a renaissance of vitamin D in dermatology? J Invest Dermatol. 2008. 128:773–775.

78. Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J Exp Med. 2009. 206:1983–1994.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download