Abstract
Eccrine porocarcinoma is a rare neoplasm that originates from the intraepidermal ductal portion of the eccrine sweat gland, and it usually occurs on the pre-existing lesion of benign eccine poroma. Its occurrence is more common in females and elderly persons. We present a case of a 44-year-old man who suffered from eccrine porocarcinoma, which developed on the right scrotum and pelvic area with metastases to the lung, adrenal gland, esophagus, ureter, and distant lymph nodes. Here we report on a unique case of eccrine porocarcinoma that developed primarily on the scrotum, which is an uncommon site, and showed rapid metastasis to the internal organs.
Eccrine porocarcinoma (EPC) is a rare cutaneous malignancy that arises from the intraepidermal ductal portion of the eccrine sweat gland. Since it was first described by Pinkus and Mehregan1. in 1963, hundreds of such cases have been reported in the literature. It occurs in both sexes, with a female predominance, and it is often seen during the sixth to eighth decades of life. It may arise de novo; however, it usually develops on the longstanding site of eccrine poroma. The lower extremities are the most common representing site, followed by the trunk, head, and upper limbs2. Twelve cases have been reported in Korea: one case showed metastasis to regional lymph nodes from the right thigh3, while 11 cases were confined to a local cutaneous area. However, a case of multiple metastases to distant organs has not yet been reported. Here we present a case of EPC that developed primarily on the scrotum, which is an uncommon site, and it showed metastasis to the internal organs in a relatively young man, and we briefly review previous reports in the Korean literature.
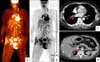
A 44-year-old man presented with painful skin lesions on the right scrotum and pelvic area, which had been present for the previous 7 months. The scrotal skin lesions were small macules and papules at the beginning; however, the lesions had shown rapid enlargement and spread to the pelvic area. His past medical history was unremarkable, except that he was a heavy drinker. On physical examination, more than half of the right scrotum was firm and ulcerated (the area measured 6.0×7.0 cm in diameter), and it was replaced by hyperemic granulation tissue with a purulent discharge (Fig. 1A, B). Erythematous nodules measuring 1.2×1.0 cm were also observed on the right pelvic area (Fig. 1C). His right leg was enlarged, compared to the left one, indicating lymphodema. The red cell count was 4.79×106 and the white cell count was 9.9×103 with 64% neutrophils. The erythrocyte sedimentation rate was 34 mm/h (normal range: 0~15 mm/h) and the C-reactive protein level was 3.19 mg/dl (normal range: 0.0~0.5 mg/dl). Other hematological and biochemical tests, including α-feto-protein (AFP), carcino-embryonic antigen (CEA), carbohydrate antigen (CA) 125, CA 19-9, and prostate specific antigen (PSA) were within normal limits. Multiple biopsy specimens showed that well-defined tumor nests composed of polygonal or cuboidal cells had invaded the dermis and subcutaneous fat layer. In addition, malignant cells demonstrated conspicuous atypia, mitoses, and necrosis. Nuclei of tumor cells were pleomorphic and vesicular on high magnification. Ductal structures were also observed (Fig. 2). On immunoperoxidase staining, EMA and cytokeratin (CK) 7 were positive in tumor nests and the cells were negative for S-100 protein, CK-20, thyroid transcription factor-1 (TTF-1), and CEA (Fig. 3). Histopathologic and immunoperoxidase findings were consistent with EPC. Computed tomography (CT) and positron emission tomography (PET) revealed metastases to both lungs, the right adrenal gland, right ureter, esophagus, and multiple lymph nodes (LN); both inguinal LNs, both external iliac LNs, the interaorto- caval LN, the paraaortic LN, and the cervical LN (Fig. 4). Combination treatment with 5-fluorouracil 1,000 mg/m2 and cisplatin 75 mg/m2 was initiated. Unfortunately, following 2 cycles, he fell victim to sepsis, and died.
EPC is a rare skin appendage tumor, with the main incidence occurring in patients more than 60 years old. The lower limbs are the most common site (44%), followed by the trunk (24%), head (18%), upper limbs (11%), and neck (3%)2. The scrotum is an extremely uncommon primary location in that only 2 such cases have been reported in the international medical literature4,5. EPC tends to be localized; however, it sometimes spreads to regional lymph nodes or adjacent skin, and it rarely metastasizes to distant organs or distant lymph nodes. Distant visceral metastases to the lung, retroperitoneum, long bones, breast, liver, mediastinum, urinary bladder, and ovary have been reported4,6. Clinical features of EPCs reported in Korea to date are summarized in Table 13,7-16. According to Shaw et al.17, all 27 cases of EPC arose from a benign eccrine poroma (BEP); however, Robson et al.2 reported occurrence of EPC in only 18% of cases of BEP. In our view, skin manifestations of our patient developed de novo because there was no preexisting lesion on the scrotum as well as on the pelvic area. In addition, EPC that arises from BEP usually shows very slow progression. This patient was relatively young and his disease showed an aggressive clinical course; the tumor developed primarily on the scrotum and showed rapid metastasis to multiple internal organs.
The histopathologic features of EPC are broad. Malignant cells usually have large and hyperchromatic nuclei. Nuclear atypia with frequent mitoses and necrosis are characteristic. Cords and nests of polygonal tumor cells penetrate to the adjacent dermis or extend into subcutaneous tissue. Histopathologically, metastatic adenocarcinoma, trabecular carcinoma, and Merkel cell carcinoma should be included in the differential diagnosis. Distinguishing EPC from metastatic adenocarcinoma, and especially adenocarcinoma of a breast or lung origin, can be difficult. However, most metastatic adenocarcinomas are positive for EMA and CEA stains. Furthermore, a positive reaction with CEA may be most helpful in differentiating between primary and secondary lesions18. In the present case, the negative reaction with CEA, the connection between the epidermis and tumor, and the absence of glandular structures are evidence for exclusion of the diagnosis of metastatic adenocarcinoma. Trabecular carcinoma and Merkel cell carcinoma also show findings comparable with EPC. Hyperchromatic, large, and irregular nuclei arranged in cord or trabecular patterns are common features of EPC and trabecular carcinoma; however, the latter shows nuclear molding. On immunoperoxidase studies, EMA and CK-7 are positive, and S-100 protein and CK-20 are negative in EPC. CEA is negative in most EPC; however, it can be positive in tumors containing well-formed ducts2. However, trabecular carcinoma or Merkel cell carcinomas express neuron-specific enolase (NSE) and CK-20, and they do not express CK-719. This present case showed positivity for EMA and CK-7 and negativity for S-100 protein, CK-20, and CEA. The negative result for CEA might be due to the paucity of ductal structures in this case.
Snow and Reizner20 suggested local recurrence and regional metastatic rates of approximately 20% and development of distant metastases in 12% of cases. Similarly, Robson et al.2 observed that 11% of patients had metastasis to distant organs; these patients have a high mortality rate (64% in the series). They suggested that the prognosis of EPC depends mainly on mitoses (more than 14 per high power field), lympho-vascular invasion, and a tumor depth of more than 7 mm. The present case was positive for all of these poor prognostic factors, and this might have induced the fatal linical course.
We experienced a rather novel case of EPC on an uncommon site and the patient presented with rapid multiple metastases. Most EPCs are localized and a few of them are metastatic. Therefore, it is essential to consider the possibility of metastases of primary EPC in the process of diagnostic evaluation of patients who are found to be normal on a physical examination.
Figures and Tables
 | Fig. 1(A, B) A firm and ulcerated right scrotum covered with hyperemic granulation tissue. (C) Multiple 1.2×1.0 cm-sized, well-demarcated nodules on the right pelvic area. |
 | Fig. 2(A) Well-defined tumor nests composed of polygonal or cuboidal cells invade the dermis and subcutaneous fat layer. Note the connection between the epidermis and tumor (H&E stain, ×40). (B) This high-grade tumor show area of necrosis (H&E stain, ×200). (C) The malignant cells have hyperchromatic, vesicular, and atypical nuclei with frequent mitoses. Ductal structures are present(H&E stain, ×400). |
References
1. Pinkus H, Mehregan AH. Epidermotropic eccrine carcinoma: a case combinig features of eccrine poroma and paget's dermatosis. Arch Dermatol. 1963. 88:597–606.
2. Robson A, Greene J, Ansari N, Kim B, Seed PT, McKee PH, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001. 25:710–720.
3. Kim JS, Ro YS, Park CK. A case of malignant eccrine poroma. Korean J Dermatol. 1998. 36:717–721.
4. Huet P, Dandurand M, Pignodel C, Guillot B. Metastasizing eccrine porocarcinoma: report of a case and review of the literature. J Am Acad Dermatol. 1996. 35:860–864.

5. Evans J, Datta MW, Goolsby M, Langenstroer P. Eccrine porocarcinoma of the scrotum. Can J Urol. 2005. 12:2722–2723.
6. de Bree E, Volalakis E, Tsetis D, Varthalitis Y, Panagiotidis J, Romanos J, et al. Treatment of advanced malignant eccrine poroma with locoregional chemotherapy. Br J Dermatol. 2005. 152:1051–1055.

7. Jung DY, Bae JH, Lee SK, Lee WW. A case of malignant eccrine poroma. Korean J Dermatol. 1999. 37:660–664.
8. Lee JH, Ahn SK, Lee ES, Lee SH. Eccrine porocaricinoma associated with seborrheic keratosis. Korean J Dermatol. 2001. 39:101–103.
9. Han KS, Choi JY, Min HG, Kim JM. A case of malignant eccrine poroma. Korean J Dermatol. 2001. 39:247–250.
10. Yoon HS, Seo SH, Kim A, Kye YC. A case of eccrine porocarcinoma. Korean J Dermatol. 2001. 39:1310–1312.
11. Kim YJ, Kim CW, Kim SY, Kim SS, Huh D, Lee JJ. A case of malignant eccrine poroma. Korean J Dermatol. 2002. 40:442–444.
12. Lee YH, Kim SH, Suh MK, Park SK, Jang TJ. Malignant eccrine poroma of the left upper eyelid resembling cutaneous horn. Korean J Dermatol. 2006. 44:1469–1471.
13. Shin JB, Ko NY, Seo SH, Kim AR, Kye YC, Kim SN. A case of eccrine porocarcinoma on the scalp. Korean J Dermatol. 2006. 44:1084–1087.
14. Oh SH, Lee WJ, Chang SE, Lee MW, Choi JH, Moon KC, et al. Two cases of eccrine porocarcinoma. Korean J Dermatol. 2007. 45:503–506.
15. Park J, Kwon H, Cho MK, Park YL, Lee SY, Lee JS, et al. A case of malignant eccrine poroma developing on the suprapubic area. Ann Dermatol. 2008. 20:37–40.

16. Choi YJ, Lee GY, Kim WS, Kim KJ. A case of eccrine porocarcinoma that showed squamous differentiation on the palm. Korean J Dermatol. 2009. 47:861–864.
17. Shaw M, McKee PH, Lowe D, Black MM. Malignant eccrine poroma: a study of twenty-seven cases. Br J Dermatol. 1982. 107:675–680.

18. Johnson WC. Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu G, editors. Metastatic carcinoma of the skin. Lever's histopathology of the skin. 2008. 10th ed. Philadelphia: Lippincott Williams & Wilkins;1154–1155.
19. Brenn T, McKee PH. McKee PH, Calonje E, Granter SR, editors. Tumors of the surface epithelium. Pathology of the skin with clinical correlations. 2005. 3rd ed. Philadelphia: Elsevier Mosby;1230–1238.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download