Abstract
Epidermolysis bullosa (EB) is a rare genetic disease that is known for continuous skin blistering caused by minor trauma. The skin blisters and bullae that develop often cause skin defects. There is no definitive treatment for EB, only symptomatic relief. We report our experience with cultured allogenic keratinocyte grafting in a newborn patient with EB simplex who had unhealed raw surfaces and was not a skin grafting candidate. The skin lesions of the patient were covered with cultured allogenic keratinocyte grafts and re-epithelialized quickly with no scarring. Allogenic keratinocyte grafting reduced pain and produced noticeable improvements in the unhealed wounds. We think that allogenic keratinocyte grafting can play an important role in the management of patients with EB simplex.
Epidermolysis bullosa (EB) is a rare genetic disease that manifests with blistering of the skin and mucous membranes following minor mechanical trauma1. Three major forms of EB have been defined using clinical and histological criteria. The simplex, junctional, and dystrophic forms of EB are characterized by loss of tissue integrity in the epidermis, at the lamina lucida or central basement membrane zone, and at the sub-lamina densa basement membrane2. In most cases, EB simplex is inherited in an autosomal dominant pattern. EB simplex is the most common type of EB, with a prevalence of approximately 1 case per 25,000 live births3.
The skin blisters and bullae that continuously develop in EB often lead to skin defects. Unhealed skin wounds lead to chronic wounds, which can give rise to squamous cell carcinoma. EB can also cause mucosal lesions, eye problems, dental problems, esophageal strictures, dysphagia, malabsorption, and many other complications.
No specific treatment is available for EB; therefore, conservative management is the mainstay treatment. Wound healing in patients with EB is challenging. An ideal dressing has not been developed, though there are a variety of suitable dressings available.
Cultured allogenic keratinocyte grafting has had encouraging results in burn patients. This prompted our interest in using it to manage patients with EB simplex.
We report our clinical experience with cultured allogenic keratinocyte grafting in a newborn patient with EB simplex who had unhealed, open 3×5 cm skin lesions and could not receive a skin graft.
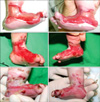
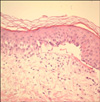
The patient, a 0-day-old boy, was immediately referred from a local clinic at birth due to multiple blisters on both feet and a wide range of skin defects. The skin defects were particularly serious on the inner surface of both feet, measured about 3×5 cm each, and displayed ongoing bleeding (Fig. 1). The patient's father had been diagnosed with EB simplex. However, he had improved with treatment and had no active skin lesions. On admission, a skin biopsy with an electromicroscopic examination and immunohistochemistry staining for cytokeratin K903 was performed, and the patient was diagnosed with EB simplex, whichshowed suprabasal separation on an electromicroscopic examination of the biopsy (Fig. 2).
He was treated with non-adhesive foam dressing materials and antibiotic ointments until post-birth day 12. However, the skin defects at birth had not epithelialized at all by day 12, and it was difficult to maintain the dressing materials given his movement. New blisters and bullae appeared secondary to mild constant irritation from the dressing materials, and the lesions enlarged and continuously bled (Fig. 1). Blisters and bullae also developed on the trunk, in the oral cavity, and on the limbs. Skin grafting could not be performed given the patient's age and disease characteristics. Thus, we considered allogenic keratinocyte grafting to promote re-epithelialization.
Kaloderm® (Tego Science, Seoul, Korea), which is cultured allogenic keratinocytes, is a biologic dressing material sheet derived from neonatal cultured forehead skin cells, which contains abundant growth factors and cytokines, and has been used to promote re-epithelialization and pain relief for second-degree burns and diabetic foot wounds.
We applied cultured allogenic keratinocyte grafts to the 3×5 cm non-epithelialized lesions on both feet at post-birth day 12 and replaced them twice at 1 week intervals without using fixation sutures. Dressings that covered the cultured allogenic keratinocyte grafts were changed daily. These overlying dressings were not fixed, so they were covered with petroleum roll gauze and sterile towels to reduce tissue trauma (Fig. 3).
Skin defects were reduced by one half, and rapid reepithelialization was confirmed on post-graft application day 7 (Fig. 1). Except for recurrent blisters, nearly total epithelialization was achieved on post-graft application day 14. The patient was discharged about 3 weeks after allogenic keratinocyte grafting, and skin lesions still occurred and were treated with ongoing dressings, but the skin defects had improved from the defects present at birth, and total epithelialization was well-maintained on the graft sites without any lesions.
EB is a group of inherited skin diseases characterized by trauma-induced bullae formation. Two main approaches can be attempted to modify wound healing in a patients with EB. One is to influence the inherent underlying abnormality and the other is to accelerate keratinocyte migration as quickly as possible.
Symptomatic treatment is needed to manage the skin, respiratory, gastrointestinal, and genitourinary systems. Management involves wound care, including lancing of blisters to prevent spread, and sterile dressings4. Nutritional support is important to promote wound healing.
Wounds in patients with EB occur as blisters or bullae. It is sometimes necessary to puncture the blister or bullae with a sterile needle to drain the fluid to prevent extension. Overlying skin should never be removed, as it acts as a biologic dressing and aides wound healing5.
Topical antibiotics should be used for short periods of time because of problems with antimicrobial resistance6. Non-adhesive foam dressings are recommended to optimize wound healing. The ideal dressing for managing EB has not been developed, although various dressings have been tried. Patients with EB are often unable to undergo surgery for skin grafting due to their age and disease characteristics. Therefore, patients with EB are treated conservatively in most cases. The recent development of bioengineered skin products has had a positive effect on wound healing in patients with EB. Treatment of difficult wounds in patients with EB may involve artificial skin substitutes such as dermal allografts, living bi-layered skin equivalents (e.g., Apligraft), biologic dressings (amniotic membrane grafting), and growth factors such as platelet derived growth factor7. Williamson et al. reported that persistent wounds were treated with Dermagraft, a fibroblastderived skin substitute, resulting in 20~100% epidermal coverage after 8 weeks in patients with epidermolysis bullosa dystrophica (DEB)7. In another report, an amniotic membrane was applied to nonhealing ulcers in three patients with DEB, and all experienced significant pain relief and developed granulation tissue within 3 days of the application8. Protein therapy, in which the missing or defective protein in patients with EB is produced in vitro using recombinant technology and applied directly to the affected skin, stem cell therapy, and gene therapy to restore normal protein production of specific structural proteins are new emerging treatments for EB9. However, these new advancements are in their initial stages, and more research into the safety and efficacy of these treatments is needed before clinical application.
The age of onset in epidermolysis bullosa simplex (EBS) is variable, with most cases manifesting at birth. Severe cases of EBS require operative management. Surgery is difficult to perform, because a skin graft makes an additional wound at the donor site characteristic of the disease. In this study, we managed the wounds of a patient with EB with cultured allogenic keratinocyte grafting, which did not require suture fixation.
Keratinocytes are abundant in the epidermis and are critical during the wound healing process. During normal wound healing, keratinocytes go to the wound base, differentiate from the basement membrane, and promote re-epithelialization. If the keratinocytes are abnormal, the wound healing process will be delayed.
In 1975, Rheinwald and Green developed a culture technique to obtain a large quantity of cultured cells from small tissues10. Since then, O'Connor et al. attempted clinical human transplants and obtained successful results11. Culturing autogenic keratinocytes requires 2~3 weeks but proliferative capacity degrades in older patients with EB. Therefore, allogenic keratinocyte culture techniques were studied beginning in the late 1980s, and cultured allogenic keratinocyte materials were developed. Autogenic keratinocytes are exposed directly to the wound bed, epithelialize, and become permanent, whereas allogenic keratinocytes occur at the wound bed temporarily and are replaced by growth and migration of the remaining keratinocytes from the wound periphery12,13. The exact wound healing mechanism of allogenic keratinocytes is unknown. It is possible that growth factors and cytokines, which are expressed and secreted in cultured allogenic keratinocytes, promote cell proliferation, migration, and create basement membrane components that effect cellular engraftment and ultimately facilitate re-epithelialization. The representative cytokines are erythroid associated factor/interleukin (IL)-1αβ, IL-6, transforming growth factor-αβ, and epidermal growth factor. Platelet derived growth factor, insulin-like growth factor, and fibroblast growth factor are secreted and accelerate wound epithelialization14-16. Similar to a membrane, cultured allogenic keratinocytes in the form of a dressing material prevent leakage of water from the wound and maintain homeostasis.
Cultured allogenic keratinocyte sheets are effective for diabetic foot wounds and second-degree or higher burns, and no reports of rejection have appeared. Despite these advantages, allogenic keratinocyte sheets do not absorb exudates and are still relatively expensive. Therefore, they have not been widely used.
The raw surface of our patient with EB simplex was covered with thawed cultured allogenic keratinocyte grafting sheets that were held in place with petroleum roll gauze and sterile surgical towel dressings. Skin defects were reduced by one half, and rapid re-epithelization was confirmed on post-graft application day 7. With the exception of recurrent bullae, nearly total epithelialization was achieved on post-graft application day 14. The use of allogenic keratinocyte grafting sheets in patients with EB presents these advantages: 1) they do not require skin grafts; thus, reducing surgical trauma; 2) they release various cytokines and growth factors that promote spontaneous rapid re-epithelialization; 3) they can relieve pain; 4) they require no anesthesia during application; 5) additional suture fixation is not needed; 6) they are very simple to use, just like a dressing material; and 7) no rejection response has been demonstrated.
In conclusion, allogenic keratinocyte grafting produced a noticeable improvement in the unhealed wounds of our patient with EB simplex by reducing trauma, and supporting epithelialization. We believe that allogenic keratinocyte grafting can play an important role in the conservative management of EB simplex.
Figures and Tables
Fig. 1
(A) Bleeding lesions (3×5 cm) on both feet of a 1 day-old boy who presented immediately after birth. (B) The medial aspects of both feet on post-birth day 12. (C) Epithelialization of the wounds on post-Kalloderm® application day 7.
References
1. Coulombe PA, Kerns ML, Fuchs E. Epidermolysis bullosa simplex: a paradigm for disorders of tissue fragility. J Clin Invest. 2009. 119:1784–1793.

2. Sawamura D, Nakano H, Matsuzaki Y. Overview of epidermolysis bullosa. J Dermatol. 2010. 37:214–219.

3. Horn HM, Tidman MJ. The clinical spectrum of epidermolysis bullosa simplex. Br J Dermatol. 2000. 142:468–472.

4. Lara-Corrales I, Arbuckle A, Zarinehbaf S, Pope E. Principles of wound care in patients with epidermolysis bullosa. Pediatr Dermatol. 2010. 27:229–237.

6. Denyer JE. Wound management for children with epidermolysis bullosa. Dermatol Clin. 2010. 28:257–264.

7. Williamson D, Coutts P, Sibbald RG. The role of dermal skin substitutes in the management of hard to heal unusual wounds. Can J Plast Surg. 2002. 10:27A–30A.
8. Hasegawa T, Mizoguchi M, Haruna K, Mizuno Y, Muramatsu S, Suga Y, et al. Amnia for intractable skin ulcers with recessive dystrophic epidermolysis bullosa: report of three cases. J Dermatol. 2007. 34:328–332.

9. Pai S, Marinkovich MP. Epidermolysis bullosa: new and emerging trends. Am J Clin Dermatol. 2002. 3:371–380.
10. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975. 6:331–343.

11. O'Connor NE, Mulliken JB, Banks-Schlegel S, Kehinde O, Green H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981. 1:75–78.
12. Compton CC, Hickerson W, Nadire K, Press W. Acceleration of skin regeneration from cultured epithelial autografts by transplantation to homograft dermis. J Burn Care Rehabil. 1993. 14:653–662.

13. Braye F, Pascal P, Bertin-Maghit M, Colpart JJ, Tissot E, Damour O. Advantages of using a bank of allogenic keratinocytes for the rapid coverage of extensive and deep second-degree burns. Med Biol Eng Comput. 2000. 38:248–252.

14. Sauder DN, Carter CS, Katz SI, Oppenheim JJ. Epidermal cell production of thymocyte activating factor (ETAF). J Invest Dermatol. 1982. 79:34–39.





 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download