Abstract
Bullous pemphigoid (BP) has a recognized association with solid organ tumors, but is relatively rare in hematological malignancies. We report a 67-year-old male who developed BP after being diagnosed with myelodysplastic syndrome and refractory anemia with excess of blast (RAEB). Skin biopsy elucidated sub-epidermal bulla using direct immunofluorescence, revealing linear C3 and IgG deposits along the basement membrane. His BP was recalcitrant to the conventional treatment and only responded to a combination of high dose oral prednisolone and azathioprine. The relative refractory nature of his condition and concurrent RAEB supports a paraneoplastic nature.
Bullous pemphigoid (BP) has a well-recognized association with malignancies though concrete evidence of its correlation remain uncertain1,2. We report our experience of a male patient with a diagnosis of myelodysplastic syndrome (MDS) and refractory anemia with excess of blast (RAEB), that presented with extensive bullous eruption. We believe this to be a rare case of RAEB presenting with paraneoplastic BP.
A 67-year-old Chinese male presented to our department with exquisitely pruritic bullae eruption about 3 months after being diagnosed with MDS with RAEB. The diagnosis was based on his bone marrow aspiration and trephine biopsy, which showed dysplastic features of all three cell lines with blast cells constituting 17% of the nucleated cells in the marrow. He also had a background history of multiple illnesses such as hypertension, ischemic heart disease, bronchial asthma and atrophic gastritis. His treatment for MDS with RAEB remained supportive with regular monthly blood transfusions as he had refused the option of chemotherapy due to his co-morbidities. The bullae started on this trunk and limbs, and later progressed to involve his mucous membrane. There was no history of new medications, supplements or other traditional medications intake within the last year.
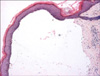
Clinically, the patient had extensive tense, hemorrhagic bullae on erythematous plaques on his limbs, with predominance over the acral regions (Fig. 1), and Nikolsky's sign was negative. In addition, there were multiple bullae on his oral mucosa, but the lesions spared his genitalia and eyes. Skin biopsy showed a sub-epidermal bulla with mild perivascular and periappendageal lymphocytic infiltration and a sprinkling of eosinophils (Fig. 2). Direct immunofluorescence revealed linear C3 and IgG deposits along the basement membrane and these confirmed the diagnosis of BP. His cutaneous condition was not controlled with oral prednisolone at 30 mg daily (0.5 mg/kg/day). The bullous eruptions were finally contained with a combination of oral prednisolone at 30 mg daily and azathioprine at 100 mg daily.
MDS is a heterogeneous group of malignant hematopoietic stem cell disorders which include six well-defined clinical entities: refractory anemia (RA), RA with multilineage dysplasia, RA with ringed sideroblasts, RAEB, MDS, unclassifiable and MDS association with del(5q)3. Cutaneous manifestations are rare in MDS with reports of Sweet's syndrome and myeloid sarcoma being the two most common, usually heralding the transformation to acute leukemia. BP is an acquired blistering dermatosis characterized by an autoimmune response to two hemidesmosomal proteins within the dermal-epidermal junction, specifically BP180 and BP230, leading to the production of IgG auto antibodies4. Although BP has been reported to be associated with malignancy, concrete evidence of its correlation and paraneoplastic significance remain uncertain1,2. Chorzelski et al. reported 11% of his BP patients to have an underlying neoplasia which was consistent with other reports2,5. However, this association is not unexpected as both of these diseases are more common among the elderly. Venning and Wojnarowska suggested that the relationship between neoplasia and BP could be due to the production of antibodies to tumor-specific antigens that may cross-react with the basement membrane zone (BMZ) leading to the development of bullae6. Other theories postulated the role of an external agent generating both the tumor and the BMZ damage, or the possibility of genetic predisposition to both conditions7. Bauduer et al. also described a case of BP as a paraneoplastic manifestation in an elderly lady who presented with synchronism between BP and transformation of a pre-existing MDS8. Our patient developed BP shortly after diagnosis of MDS with RAEB. This remarkable coincidence was reported by Modiano et al. who postulated that the tumor infiltrate could have produced antigenic determinants leading to the development of BP9. The typical distribution of BP on flexural skin areas with infrequent oral involvement is in contrast to our patient who presented with bullae and erosions predominantly in his distal extremities and oral cavity. This again suggested a more severe form of disease, occurring in association with RAEB.
The management of patients with BP is largely dependent on the degree of cutaneous involvement and rate of disease progression. This condition is generally quite responsive to oral steroid therapy, with up to 75% of patients achieving prolonged clinical remission without further therapy2,10. Patients with localized disease may sometimes be managed successfully with potent topical steroids2,11. Those with moderate diseases are often treated with oral prednisolone. Steroid sparing agents are only added for patients with extensive disease that are not responding well to prednisolone monotherapy. Our patient, who had severe disease, required two systemic agents to arrest his cutaneous eruption. This is in parallel with the nature of paraneoplastic disease, whereby the progression of his cutaneous lesions follows that of his MDS and RAEB. Hence, with the absence of treatment for the latter, his BP was more recalcitrant to treatment.
In summary, the simultaneous occurrence of BP in the presence of MDS with RAEB suggests that BP may be a rare paraneoplastic syndrome, although this association has yet to be defined.
Figures and Tables
References
1. Iuliano L, Micheletta F, Natoli S. Bullous pemphigoid: an unusual and insidious presentation of breast cancer. Clin Oncol (R Coll Radiol). 2003. 15:505.

2. Ogawa H, Sakuma M, Morioka S, Kitamura K, Sasai Y, Imamura S, et al. The incidence of internal malignancies in pemphigus and bullous pemphigoid in Japan. J Dermatol Sci. 1995. 9:136–141.

3. Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999. 17:3835–3849.

4. Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol. 2007. 33:67–77.

6. Venning VA, Wojnarowska F. The association of bullous pemphigoid and malignant disease: a case control study. Br J Dermatol. 1990. 123:439–445.

7. Iranzo P, López I, Robles MT, Mascaró JM Jr, Campo E, Herrero C. Bullous pemphigoid associated with mantle cell lymphoma. Arch Dermatol. 2004. 140:1496–1499.

8. Bauduer F, Barteau A, Truchet S, Massot-Bordenave J, Ducout L. Bullous pemphigoid associated with the transformation of a preexisting myelodysplastic syndrome. Leuk Lymphoma. 1999. 32:399–400.

9. Modiano P, Reichert S, Barbaud A, Bernard P, Weber M, Schmutz JL. Bullous pemphigoid in association with cutaneous lesions specific to a myelodysplastic syndrome. Br J Dermatol. 1997. 136:402–405.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download