Abstract
Over the past years, hydrocolloid dressings have been introduced routinely in the treatment of various types of wounds. They provide a moist environment promoting autolytic debridement, and stimulate angiogenesis. However, long-term application often leads to inflammation of the skin in the immediate area of the ulcer, causing irritant dermatitis in many cases, but sometimes also leads to contact sensitization. A 32 year-old woman burnt herself by an iron, and presented to our clinic and was treated with Duoderm extrathin®. Nine days later, she again presented with an erythematous oozing patch with edema, and allergic contact dermatitis was suspected. A patch test (TRUE test) was performed and a positive reaction to colophonium was obtained. Duoderm extrathin® contains hydrogenated rosin (colophonium) as the tackifying agent, so we could diagnose this case as allergic contact dermatitis due to the hydrogenated rosin in Duoderm extrathin®. We report another case of allergic contact dermatitis due to Duoderm extrathin® in a 32 year-old woman.
Hydrocolloid dressings have been introduced routinely in the treatment of various types of wounds. They provide a moist environment promoting autolytic debridement, and stimulate angiogenesis, thus ultimately promoting wound healing1. The most widely used product of hydrocolloid dressing agents is Duoderm extrathin®. However, hydrocolloid dressings can be the cause of irritant contact dermatitis and can also cause allergic contact dermatitis. In Korean dermatological literature, there are 2 cases of allergic contact dermatitis due to Duoderm extrathin® reported2,3. One was due to Duoderm CGF®3 and the other was due to Duoderm extrathin®2. In foreign literature, over 40 cases of allergic contact dermatitis due to hydrocolloid dressings were reported1,4-11. Herein, we report another case of allergic contact dermatitis due to Duoderm extrathin®.
A 32 year-old Korean woman presented with an erythematous pruritic patch on the shin after applying Duoderm extrathin®, due to a burn by an iron. She first came to our clinic due to this burn and then had it dressed with Duoderm extrathin®. Two days later, the pruritus started and it gradually became more severe. Also, an erythematous patch was observed around the dressing site and she removed the Duoderm extrathin®. She had already experienced the same type of episode two years prior. Nine days after removing the Duoderm extrathin®, the pruritus and erythema remained, so she came to our clinic.
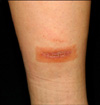
Physical examination showed a well-demarcated erythematous vesicular patch on the Duoderm extrathin® attachment site (Fig. 1). Based on the same episode after dressing with Duoderm extrathin®, and the ongoing symptoms of pruritus and erythema after removing the Duoderm extrathin®, allergic contact dermatitis due to the Duoderm extrathin® was highly suspicious. Thus, a patch test (TRUE test) was performed to confirm the diagnosis and to identify the causing agent.
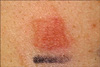
A positive reaction to colophony was observed in the patch test. Ninety-six hours later, an erythematous patch with vesicles and papules was seen on the colophony site (Fig. 2).
Finally, allergic contact dermatitis due to Duoderm extrathin® was diagnosed based on the clinical manifestations and the patch test results.
Duoderm extrathin® is the most widely used product of hydrocolloid dressing agents. Duoderm CGF® is used when the amount of exudate is large. The outermost foam layer of Duoderm extrathin® consists of polyurethane and the inner adhesive skin contact layer consists of pectin, gelatin, and sodium carboxymethylcellulose. The pentaerythritol ester of hydrogenated colophonium, Pentalyn, is used as the tackifying agent of Duoderm extrathin® in the adhesive skin contact layer, and it is the most responsible allergen to cause allergic contact dermatitis due to Duoderm1.
Colophonium is a natural product obtained mainly from pine trees and has a complex composition consisting mainly of resin acids, of which abietic acid and dehydroabietic acid are the major components. Furthermore, colophonium is used in different modified forms: hydrogenated, disproportionated, pentaerythritol esterified, glycerol esterified, maleic anhydride modified, fumaric acid modified, formaldehyde modified, polymerized, or in salt form12.
So far, 44 cases of allergic contact dermatitis caused by hydrocolloid dressings have been reported in domestic and foreign dermatological literature (Table 1). Duoderm is called various names in different countries. It is called Duoderm in America, and most European and Asian countries, and Granuflex in the UK and South africa, Duoactive in Japan, and Varihesive in Germany. The old product 'Duoderm' used polyisobutylene as the tackifying agent, but the new products 'Duoderm extrathin® and Duoderm CGF®, contain pentanyl as the tackifying agent. In almost half of the cases of allergic contact dermatitis due to Duoderm, 20 out of 44, the responsible allergens were modified colophonium, especially pentalyn and pentaerythritol ester of the hydrogenated colophonium. In 16 of 44 cases, the responsible allergens were not identified. In these cases, the reaction may be caused by skin irritation or may be an allergic contact reaction to potentially contaminating unpolymerized substances of elastomers in the hydrocolloid dressing12. Since in these cases the results of patch tests to both colophonium and pentalyn were negative, there may be another potential allergen.
Our case showed a positive reaction to colophony in the patch test (TRUE test). So we can conclude this case was allergic contact dermatitis due to colophonium in the Duoderm. There have been 2 cases of allergic contact dermatitis due to Duoderm extrathin® reported in Korea. In both cases, a routine patch test was not performed.
Dermatologists and general physicians should pay attention to the possibility of allergic contact dermatitis to hydrocolloid dressings. Furthermore, in the future, the complete composition of hydrocolloid dressing should be legally required on the label.
Figures and Tables
References
1. Pereira TM, Flour M, Goossens A. Allergic contact dermatitis from modified colophonium in wound dressings. Contact Dermatitis. 2007. 56:5–9.

2. Seo JK, Lee HJ, Hong SK, Lee D, Choi HC, Sung HS, et al. A case of allergic contact dermatitis to duoderm extrathin(R) dressing. Korean J Dermatol. 2009. 47:220–222.
3. Lee JH, Kim SC. A case of allergic contact dermatitis from duoderm hydrocolloid dressing. Korean J Dermatol. 2000. 38:1256–1257.
4. Mallon E, Powell SM. Allergic contact dermatitis from Granuflex hydrocolloid dressing. Contact Dermatitis. 1994. 30:110–111.

5. Arnold WP, Rath R, van der valk PGM. Allergic contact dermatitis from Duoderm E hydrocolloid dressing. Ned Tijdschr Dermatol Venereol. 1995. 5:116–117.
6. Schliz M, Rauterberg A, Weiss J. Allergic contact dermatitis from hydrocolloid dressings. Contact Dermatitis. 1996. 34:146–147.

7. Molin L, Stymme B, Start HU, Eriksson IL. Allergic contact dermatitis induced by tackifying agents in hydrocolloid dressing. J Eur Acad Dermatol Venereol. 1996. 7:Suppl.2. S142–S143.
8. Parslew R, Evans S, King CM. Allergic contact dermatitis from polyisobutylene in stoma bags. Contact Dermatitis. 1996. 35:178–179.

9. Sasseville D, Saber M, Lessard L. Allergic contact dermatitis from tincture of benzoin with multiple concomitant reactions. Contact Dermatitis. 2009. 61:358–360.

11. Grange-Prunier A, Couilliet D, Grange F, Guillaume JC. Allergic contact dermatitis to the Comfeel hydrocolloid dressing. Ann Dermatol Venereol. 2002. 129:725–727.
12. Timmer-de Mik L, Toonstra J. Allergy to hydrocolloid dressings. Contact Dermatitis. 2008. 58:124–125.

13. Lim KS, Tang MB, Goon AT, Leow YH. Contact sensitization in patients with chronic venous leg ulcers in Singapore. Contact Dermatitis. 2007. 56:94–98.

14. Koo FP, Piletta-Zanin P, Politta-Sanchez S, Milingou M, Saurat JH. Allergic contact dermatitis to carboxymethylcellulose in Comfeel hydrocolloid dressing. Contact Dermatitis. 2008. 58:375–376.

15. Körber A, Kohaus S, Geisheimer M, Grabbe S, Dissemond J. Allergic contact dermatitis from a hydrocolloid dressing due to colophony sensitization. Hautarzt. 2006. 57:242–245.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download