Abstract
Acute generalized exanthematous pustulosis (AGEP) is manifested by rapid development of many sterile, nonfollicular pustules on a background of edematous erythema. More than 90 percent of AGEP are induced by medication, mostly antibiotics. Drug patch test can be helpful in the diagnosis of AGEP. This paper reports the first case of celecoxib-induced AGEP confirmed by patch test in Korean literature.
Acute generalized exanthematous pustulosis (AGEP) is an uncommon eruption characterized by acute occurrence of many non-follicular sterile pustules on edematous erythema. More than 90 percent of AGEP are induced by medication and most of them are antibiotics1. Other etiologic agents include viral infection, mercury and lacquer2,3. Celecoxib is a non-steroidal anti-inflammatory drug which inhibits cyclooxygenase-2 (COX-2) and prescribed for control of pain. In English literature, three cases of celecoxib induced AGEP had been reported4-6. We report a first case of AGEP induced by celecoxib confirmed by patch test in Korean literature.
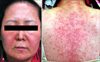
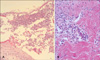
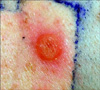
A 53-year-old Korean woman without any history of allergy and psoriasis presented with disseminated pustules on the face and trunk. The patient had fever and itching. From two weeks before visiting our hospital, she started to take celecoxib for her shoulder pain. Ten days after taking medication, erythematous skin eruption with high fever had developed, and she stopped taking celecoxib. After two days, numerous small pustules developed in these erythematous lesions. Physical examination revealed many tiny non-follicular pustules on the face (Fig. 1A) and trunk (Fig. 1B). The skin lesion was accompanied by leukocytosis (10.17×103/µl) with elevated neutrophil counts (8.39×103/Ml) and C-reactive protein levels (7.32 mg/dl). No microrganism was identified in blood cultures and pustule cultures. Skin biopsy perfomed on the patient's back revealed subconeal pustules, spongiosis in the epidermis, papillary dermal edema and perivascular infiltration of lymphocytes, neutrophils and some eosinophils on papillary dermis (Fig. 2). On admission, the patient was treated with methylprednisolone 40 mg twice a day and improved within seven days. AGEP induced by celecoxib was the most likely suspect. After three months, a drug patch test was performed with celecoxib diluted at 5 percent in normal saline and in petrolatum. To determine whether the constituents of the capsule caused AGEP, constituents of the capsule were diluted at 5 percent in normal saline and in petrolatum; these were also included in patch test. At the same time, several other antibiotics and non-steroidal anti-inflammatory drugs were tested, but sulfonamide drugs were not included. To rule out false-positive reactions, two healthy persons were also tested. The patient showed strong vesicular reaction to celecoxib diluted at 5 percent in normal saline (Fig. 3) and erythematous reaction to celecoxib diluted at 5 percent in petrolatum. The result for other drugs was negative. It was concluded that celecoxib was the cause of AGEP.
When Baker and Ryan7 reported 104 cases of pustular psoriasis in 1968, they found five patients who had no history of psoriasis, rapidly progressing and with quick improvement. In 1980 Beylot et al.8 introduced the term AGEF.
AGEP is a rare and severe pustular reaction of drug eruption characterized by acute, extensive, non-follicular pustules, accompanied by high fever and leukocytosis. Mild oral mucous membrane involvement may occur in approximately 20 percent of AGEP. Pustules resolve spontaneously within a few (~4 to 10) days and are followed by postpustular, pin-point desquamation. Undoubtedly, AGEP is improved by discontinuing the culprit medication and antibiotics are unnecessary unless there are signs of infection. Because AGEP is a self-limiting disease, systemic steroid treatment is not always required1.
Currently, several mechanisms of AGEP have been proposed. Britschgi et al.4 suggested the involvement of a drug-specific T cell, which produces interleukin (IL)-8 and IL-5. The other mechanisms are the formation of antigenantibody complex by viral infection or medication that causes activating of the complement system2.
Drug patch tests can be helpful in the defining the cause of AGEP9. Based on the fact that the patch test shows positive results, AGEP is considered as a delayed type of hypersensitivity reaction. However, the usefulness of the drug patch test is dependent on the tested drug. Because of the possibility of false negative results, the drug patch test should be performed with high concentrations of the commercialized form of the medication. Thirty percent is the maximum concentration possible to get a uniform dilution in petrolatum or in water. In drug patch tests for celecoxib with concentration higher than 10 percent, irritant reactions were reported9. To avoid false positive results by irritation, the patch test was performed with celecoxib diluted at 5 percent in normal saline and petrolatum and two healthy persons were also tested. The patient showed positive result and the two healthy persons showed negative results.
Celecoxib, a selective COX-2 inhibitor, is a non-steroidal anti-inflammatory drug for the reduction of pain. A number of skin adverse effects of celecoxib have been reported, including urticaria, anaphylasis, angioedema, erythema multiforme, fixed drug eruption, vasculitis, Stevens-Johnson syndrome, sweet's syndrome, toxic epidermal necrolysis, and AGEP5. Since celecoxib contains a sulfonamide substituent, it has the potential for cross-reacting with other sulfonamide antibiotics. However, some authors disagree as to the presence of structural differences between sulfonamide antibiotics with aromatic amines and other sulfonamide containing drugs with non-aromatic amines5,6. In the case, the cross reaction had not been evaluated.
To the authors' knowledge, this is the first report of celecoxib-induced AGEP confirmed by patch test in Korean literature. Drug patch tests can be helpful in the defining the cause of AGEP, but there is no standardization for the test. Therefore, a sufficient literature review is essential for drug patch tests.
Figures and Tables
Fig. 1
(A) Numerous tiny nonfollicular pustules on the face. (B) A hundred of non-follicular pustules on the back.
References
1. Sidoroff A, Halevy S, Bavinck JN, Vaillant L, Roujeau JC. Acute generalized exanthematous pustulosis (AGEP)--a clinical reaction pattern. J Cutan Pathol. 2001. 28:113–119.

2. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003. 139:1181–1183.

3. Choi MJ, Kim HS, Park HJ, Park CJ, Lee JD, Lee JY, et al. Clinicopathologic manifestations of 36 korean patients with acute generalized exanthematous pustulosis: a case series and review of the literature. Ann Dermatol. 2010. 22:163–169.

4. Britschgi M, Steiner UC, Schmid S, Depta JP, Senti G, Bircher A, et al. T-cell involvement in drug-induced acute generalized exanthematous pustulosis. J Clin Invest. 2001. 107:1433–1441.

5. Goeschke B, Braathen LR. Acute generalized exanthematic pustulosis: a case and an overview of side effects affecting the skin caused by celecoxib and other COX-2 inhibitors reported so far. Dermatology. 2004. 209:53–56.

6. Yang CC, Lee JY, Chen WC. Acute generalized exanthematous pustulosis caused by celecoxib. J Formos Med Assoc. 2004. 103:555–557.
7. Baker H, Ryan TJ. Generalized pustular psoriasis. A clinical and epidemiological study of 104 cases. Br J Dermatol. 1968. 80:771–793.
8. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) (author's transl). Ann Dermatol Venereol. 1980. 107:37–48.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download