Abstract
Imatinib mesylate (Gleevec™, STI571), a selective inhibitor of BCR-ABL, c-Kit, and platelet-derived factor receptor, has been used to treat chronic myelogenous leukemia (CML) and gastrointestinal stromal tumors. Although its use has been associated with various adverse cutaneous reactions, pityriasis rosea-like drug eruptions are rare. Here, we report a case of pityriasis rosea-like drug eruption that developed following the administration of imatinib mesylate to treat CML.
Imatinib mesylate (Gleevec™, STI571) functions as a competitive inhibitor at the adenosine triphosphate binding site of the enzyme, preventing the tyrosine phosphorylation of proteins involved in BCR-ABL signal transduction1. It has been approved for the treatment of chronic myeloid leukemia and gastrointestinal stromal tumor and has been found efficacious in other neoplastic diseases. Although its use has been associated with various adverse cutaneous reactions, pityriasis rosea-like drug eruptions are rare. Here, we describe a 74-year-old female patient who presented with pityriasis rosea-like drug eruption that developed following the administration of imatinib mesylate to treat chronic myelogenous leukemia (CML).
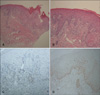
A 74-year-old female patient was diagnosed with CML and treated with imatinib mesylate (Gleevec™) (400 mg/day). After 1 month of treatment, she developed edema in her face and legs, which improved following treatment with diuretics. After 2 months of treatment, she developed non-pruritic, variably-sized, erythematous scaly patches along the cleavage lines of the trunk and extremities (Fig. 1). Other than imatinib mesylate and diuretics, the patient was taking no other prescribed medicines. Laboratory findings revealed that she was suffering from pancytopenia, and a serological test for syphilis was negative. Histological analyses of the biopsy from the trunk revealed perivascular inflammatory infiltration by lymphocytes, a small number of eosinophils, and extravasation of red blood cells into the superficial dermis. The epidermis was characterized by parakeratosis, acanthosis, spongiosis, lymphocyte exocytosis, scattered necrotic keratinocytes, and vacuolar alteration of keratinocytes in the basal layer of the epidermis (Fig. 2A, B). The intraepidermal lymphocytes were primarily CD8+ T cells, and the dermal perivascular and interstitial lymphocytes were primarily CD4+ T cells (Fig. 2C, D).
Discontinuation of imatinib mesylate therapy and the simultaneous initiation of systemic antihistamine and topical corticosteroid therapy brought about complete remission of the cutaneous lesions within 2 weeks. When imatinib mesylate therapy was recommenced (400 mg/day), a cutaneous rash reappeared 7 days later. Imatinib mesylate was subsequently replaced with nilotinib, a second-generation BCR-ABL tyrosine kinase inhibitor. The patient has since suffered no recurrence of the skin rash.
Based on the drug history, clinical presentation, histopathological findings, and clinical course, our patient was diagnosed with pityriasis rosea-like drug eruption induced by imatinib mesylate.
Imatinib mesylate (Gleevec™, STI571), a selective inhibitor of BCR-ABL, c-kit and platelet-derived factor receptor (PDGFR), has been used to treat CML and gastrointestinal stromal tumors. Adverse reactions induced by imatinib mesylate include hematologic responses and non-hematologic responses, including nausea, vomiting, diarrhea, myalgia, edema, and skin rash1,2. The cutaneous adverse reactions commonly induced by imatinib mesylate (Gleevec™) may be non-specific such as exanthematous rash, pruritus, and edema, or may show eruptions with distinctive clinical characteristics, as in Stevens-Johnson syndrome3, acute generalized exanthematous pustulosis4,5, exfoliative dermatitis6-8, psoriasiform drug eruption or exacerbation of psoriasis9,10, pityriasis rosea-like eruption11,12, and oral lichenoid reaction13,14.
Although the shin area showed similar features to psoriasis, the general morphology of cutaneous lesion were thin, fine scaly patches, not thick, crusted plaques, which does not correlate with psoriasis. Histologic examination showed pityriasis rosea-like features, such as patchy parakeratosis, focal spongiosis with lymphocytic exocytosis, superficial perivascular lymphocytic infiltrate with red cell extravasation rather than characteristic features of psoriasis, or psoriasiform drug eruptions, such as neutrophilc infiltration and confluent parakeratosis. In addition, clinically typical pityriasis rosea like drug eruption may be characterized by a more psoriasiform histology. Therefore, our patient was diagnosis with pityriasis rosea or pityriasis rosea-like drug eruption. At first glance, we could not differentiate between pityriasis rosea-like drug eruption and classic pityriasis rosea, which was not associated with imatinib mesylate. However, we concluded that the eruptions were likely caused by imatinib mesylate for several reasons. First, histopathological features were different form classic pityriasis rosea. The CD8+ T cells infiltrating the epidermis and CD4+ T cells infiltrating the dermis were consistent with an allergic drug reaction15. In classic pityriasis rosea, the intraepidermal and dermal T cells are typically CD4+. Second, there was a strong correlation between the time course of the reaction and the administration of imatinib mesylate, as the skin rash subsided following the discontinuation of treatment using this medication. Third, the re-administration of imatinib mesylate caused the skin reaction to flare up again, which provided further evidence linking the 2 events.
Pityriasis rosea-like drug eruptions induced by imatinib mesylate are very rare. Only 4 cases have been documented in 2 reports11,12. Konstantopoulos et al.11 described a case of typical pityriasis rosea associated with imatinib mesylate, but the authors did not present images of the affected skin and did not perform a biopsy. Re-challenge with imatinib mesylate was not possible, so the diagnosis was tentative. Therefore, we consider the report of Brazzelli et al.12 to be the first to confirm this diagnosis. All three 3 they presented showed clinical features of atypical pityriasis rosea and histopathological evidence of non-specific drug eruptions, suggesting a diagnosis of pityriasis rosea-like eruptions induced by imatinib mesylate.
Although the pathophysiology of the adverse cutaneous reactions is unclear, the relationship between incidence and dosage suggests that these reactions may stem from the pharmacological effects of imatinib mesylate, rather than a hypersensitivity reaction2,16. The imatinib mesylate target, c-Kit, is normally expressed in basal epidermal keratinocytes, melanocytes, tissue mast cells, breast epithelial cells, and dermal sweat glands, among other cells17. The pathophysiology of adverse skin reactions may be attributable to the inhibition of c-Kit and/or PDGFR tyrosine kinase activity in these skin cells2.
Management of mild to moderate skin eruptions induced by imatinib mesylate is usually achieved by discontinuing the drug treatment. In patients presenting with severe rash or peripheral eosinophilia, oral steroid treatment (e.g., prednisolone, 1 mg/kg) is often helpful18. For cases in which the re-administration of imatinib mesylate is necessary to treat the primary disorder (e.g., leukemia), it may be possible to continue imatinib mesylate treatment with concomitant short-term steroid therapy (prednisolone, 5 mg/day). Alternatively, starting with a low dose of (100 mg/day) and gradually increasing the dosage may be successful19. On a case-by-case basis, careful management can allow patients who develop mild to moderate skin rashes on imatinib mesylate to continue with the treatment. In our case, when imatinib mesylate was re-administered at a same dose, the cutaneous rash reappeared. As a consequence, imatinib mesylate was replaced with nilotinib, a second-generation BCR-ABL tyrosine kinase inhibitor. Our patient has since suffered no recurrence of the skin rash.
Various adverse events linked to the administration of imatinib mesylate have been reported. Here, we presented a rare case of pityriasis rosea-like drug eruption that developed following the administration of imatinib mesylate to treat CML.
Figures and Tables
Fig. 1
(A~D) Erythematous scaly patches are seen distributed along the cleavage lines of the trunk and extremities.
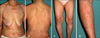
Fig. 2
(A, B) Acanthosis, parakeratosis, spongiosis, lymphocyte exocytosis, and the presence of necrotic keratinocytes in the epidermis. Vacuolar alterations at the dermo-epidermal junction are evident. Infiltration by lymphocytes and a smaller number of eosinophils, and extravasation of red blood cells are observed in the superficial dermis (H&E; ×40, ×100). (C, D) The intraepidermal lymphocytes are primarily CD8+ T cells; and the dermal perivascular and interstitial lymphocytes, primarily CD4+ T cells (C: CD4, D: CD8, ×100).
References
1. Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001. 344:1031–1037.

2. Valeyrie L, Bastuji-Garin S, Revuz J, Bachot N, Wechsler J, Berthaud P, et al. Adverse cutaneous reactions to imatinib (STI571) in Philadelphia chromosome-positive leukemias: a prospective study of 54 patients. J Am Acad Dermatol. 2003. 48:201–206.

3. Hsiao LT, Chung HM, Lin JT, Chiou TJ, Liu JH, Fan FS, et al. Stevens-Johnson syndrome after treatment with STI571: a case report. Br J Haematol. 2002. 117:620–622.

4. Brouard MC, Prins C, Mach-Pascual S, Saurat JH. Acute generalized exanthematous pustulosis associated with STI-571 in a patient with chronic myeloid leukemia. Dermatology. 2001. 203:57–59.

5. Schwarz M, Kreuzer KA, Baskaynak G, Dörken B, le Coutre P. Imatinib-induced acute generalized exanthematous pustulosis (AGEP) in two patients with chronic myeloid leukemia. Eur J Haematol. 2002. 69:254–256.

6. Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001. 344:1038–1042.

7. Yoo KH, Rho YK, Kim JY, Li K, Seo SJ, Hong CK. A case of drug eruption with localized exfoliative dermatitis induced by imatinib mesylate. Korean J Dermatol. 2008. 46:1435–1438.
8. Joo YH, Min SU, Lee DH, Suh DH. A case of drug eruption with periorbital edema and exfoliative dermatitis induced by imatinib mesylate (STI571, Gleevec(TM)). Korean J Dermatol. 2007. 45:194–196.
9. Bae YI, Yun SJ, Lee JB, Kim SJ, Lee SC, Won YH. A case of psoriasiform drug eruption induced by imatinib mesylate (Gleevec™). Korean J Dermatol. 2009. 47:722–725.
10. Woo SM, Huh CH, Park KC, Youn SW. Exacerbation of psoriasis in a chronic myelogenous leukemia patient treated with imatinib. J Dermatol. 2007. 34:724–726.

11. Konstantopoulos K, Papadogianni A, Dimopoulou M, Kourelis C, Meletis J. Pityriasis rosea associated with imatinib (STI571, Gleevec). Dermatology. 2002. 205:172–173.

12. Brazzelli V, Prestinari F, Roveda E, Barbagallo T, Bellani E, Vassallo C, et al. Pityriasis rosea-like eruption during treatment with imatinib mesylate: description of 3 cases. J Am Acad Dermatol. 2005. 53:5 Suppl 1. S240–S243.

13. Lim DS, Muir J. Oral lichenoid reaction to imatinib (STI 571, Gleevec). Dermatology. 2002. 205:169–171.

14. Basso FG, Boer CC, Corrêa ME, Torrezan M, Cintra ML, de Magalhães MH, et al. Skin and oral lesions associated to imatinib mesylate therapy. Support Care Cancer. 2009. 17:465–468.

15. Rozieres A, Vocanson M, Saïd BB, Nosbaum A, Nicolas JF. Role of T cells in nonimmediate allergic drug reactions. Curr Opin Allergy Clin Immunol. 2009. 9:305–310.

17. Lammie A, Drobnjak M, Gerald W, Saad A, Cote R, Cordon-Cardo C. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem. 1994. 42:1417–1425.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download