Abstract
Basal cell nevus syndrome (BCNS), or Gorlin Syndrome, is an autosomal dominant disorder, characterized by multiple developmental abnormalities and associated with germline mutations in the PTCH gene. Patients show multiple and early onset basal cell carcinomas (BCCs) in skin, odontogeniccysts in the jaw, pits on palms and soles, medulloblastoma, hypertelorism, and calcification of the falx cerebri. Clinical features of BCCs in these patients are indistinguishable from ordinary BCCs. However, some patients show variable histologic findings in subtypes of BCCs, and only one case associated with several histologic types of BCCs in the syndrome has been reported in Korea. We present a case of BCNS characterized by multiple BCCs, odontogenic keratocysts, multiple palmar pits, and calcified falx cerebri. Histopathologic findings of BCCs showed several patterns, which were nodular, superficial, and pigmented types.
Basal cell nevus syndrome (BCNS), also known as Gorlin syndrome or nevoid basal cell carcinoma syndrome (NBCCS), is an uncommon disorder, which is inherited as an autosomal dominant trait1. Patients with this syndrome often have anomalies of multiple organs, such as abnormalities of the skin, skeleton, and nervous system. This disorder is characterized by variable clinical manifestations, including multiple Basal cell carcinomas (BCCs); odontogenic keratocysts of the jaw or a polyostotic bone cyst; pitted depression on the palms and soles; skeletal anomalies, including bifid, fused rib or vertebrae, and scoliosis; congenital malformation, including cleft lip or palate, microphthalmos, and polydactyly; central nervous system abnormalities, including lamellar or early calcification of the falx cerebri, and medulloblastoma in childhood2,3. Germline mutations in the PTCH gene on chromosome 9q23 have been found in 35~50% of patients. The PTCH gene is known to be important in developmental abnormalities, segmentation, and growth regulation. A deletion or inactivation of this gene is believed to cause the disorder because the PTCH gene plays a role as a tumor suppressor gene4,5. BCCs have various clinical and histologic subtypes, which include nodular or solid, cystic, micronodular, pigmented, adenoid, superficial, morpheaform or sclerosing, fibroepithelioma of Pinkus, and mixed type. Approximately one third of patients with this syndrome show various types of BCCs6-8.
However, only one case of BCNS with diverse histologic findings of BCCs has been reported in Korea9. Herein, we report on an interesting case of BCNS in a 68-year-old male who presented with several histologic types of BCCs.
A 68-year-old man presented with multiple pigmented papulo-nodular lesions on the face, back, and thigh for several years. He had no subjective symptoms and no other medical history, and there were no other affected relatives having BCNS. Physical examination revealed a pea-sized, pigmented nodule on the right nasolabial fold (Fig. 1A), with erythematous, hyperpigmented patches, multiple-sized pigmented nevi on his back (Fig. 1B, C), and a black colored and ulcerative plaque on the right thigh (Fig. 1D). He had multiple pits on his palms (Fig. 2A) and mild ocular hypertelorism. Skull X-ray showed intracranial calcification of the falx cerebri (Fig. 2B), and a cystic lesion of the left maxillary sinus revealed was by facial CT (Fig. 2C). It was proven to be an odontogenic keratocyst by histologic finding.
Histopathological examination revealed several histological variants of BCCs; nasolabial fold (nodular, Fig. 3A), back (superficial, Fig. 3B), and thigh (mixed pattern of nodular, superficial, and pigmented type, Fig. 3C). Multiple BCCs in the skin and the odontogenic cyst were treated with surgical excision, and no further relapse was observed.
BCNS, or Gorlin syndrome, was first described by Jarisch and White in 189410,11 and was later established as a syndrome by Gorlin and Goltz in 196012. This disease was initially characterized by the presence of triads of multiple basal cell carcinomas, odontogenic keratocysts of the jaw, and skeletal anomalies, including bifid rib and scoliosis12. Supplementary clinical manifestations of intracranial ectopic calcification and palmar or plantar pits were also observed, and these symptoms were added to the major criteria for diagnosis13.
While it is known to be a familial and an autosomal dominant syndrome, the exact pathogenesis is not understood. The syndrome exhibits high penetrance and variable expressivity1. The genetic abnormality underlying BCNS is caused by mutations in the PTCH gene and by downregulation of the sonic hedgehog signaling pathway. The PTCH gene acts as a tumor suppressor gene, and has been mapped to the long arm of chromosome 9 q22.3-q31. Loss of function of the gene induces abnormal growth and development of normal tissues, which leads to the syndrome4,5.
Diagnosis of BCNS can be made when two of the five major criteria, or one major and two minor criteria are present14,15. Major criteria of this syndrome include the following: (1) multiple (two or more) basal cell carcinomas or one BCC under 30 years, or ten or more basal cell nevi; (2) odontogenic keratocysts of the jaw; (3) multiple (three or more) palmar or plantar pits; (4) ectopic calcification, such as lamellar calcification of the falx cerebri; and (5) family history of Gorlin's syndrome. Minor manifestations include the following: (1) skeletal anomalies, such as bifid, fused rib or vertebrae, marked syndactyly of the digits, and scoliosis; (2) congenital malformation, including cleft lip or palate, macrocephaly, hypertelorism, and frontal bossing; (3) medulloblastoma in young children; and (4) cardiac or ovarian fibroma. In addition, Lo Muzio et al.1 suggested a specific clinical protocol for examination of patients with suspicion of this syndrome. Cases of BCNS in advanced age have occasionally been reported. However, since lesions were treated at any point where symptoms not compatible with BCNS criteria were observed, those cases did not actually occur in old age. Most patients showed multiple basal cell carcinomas, which were regarded as seborrheic keratosis, pigmented nevus, and melanocytic nevus. In addition, the odontogenic cyst, palmoplantar pits, and other clinical findings were treated when symptoms developed; therefore, it is difficult to demonstrate that BCNS occurs in older patients.
BCCs in this syndrome have been found in 50 to 75% of patients and they most often appear between puberty and 35 years of age. The number of lesions varies from a few to several thousand and ranges in size from 1 to 30 mm in diameter3,5,16. Pits of palms and soles are distinct features of this syndrome and these lesions are 2~3 mm in diameter and 1~3 mm in depth9. They may occur in 85% of patients with BCNS who are over the age of 20 years. Histologically, loss of the horny layer, thin granular layer, vacuolization of the malphigian layer, and irregular elongation of the epidermal rete ridge are observed17.
The histopathology of BCCs in this syndrome cannot be differentiated from that of sporadic BCCs3,7. In addition, approximately 30% of patients have two or more histologic sub-types of BCCs with patterns of superficial, solid or nodular, cystic, adenoid, morpheaform or sclerosing, and fibroepithelioma of Pinkus6-8. Several cases of BCNS have been reported in Korea5,9,16-18; however, only one reported case has shown various histopathologic types of BCCs in the syndrome9. According to this report, two cases of BCNS presented with superficial, nodular, and follicular or infundibulocystic patterns in histologic types of BCCs.
BCNS must be distinguished from rare disorders, such as linear unilateral nevoid BCCs with comedones, Bazex syndrome, and Rombo syndrome17. Bazex syndrome is an X-linked dominant syndrome, characterized by multiple BCCs of the face, hypohidrosis, hypotrichosis, and follicular atroderma of the extremities. Rombo syndrome is a dominantly inherited disorder with multiple BCCs, hypotrichosis, vermiculate atrophoderma, milia, trichoepitheliomas, and peripheral cyanosis19.
Early diagnosis and proper management of this syndrome are important, since it has malignant transformation. Management of patients with BCNS involves genetic counseling, imaging studies, biopsy of suspected skin lesions, and treatment of related findings1. Surgical treatments, including cryotherapy, electrodessication and curettage, simple excision, and Mohs micrographic surgery are used for most lesions. Topical therapy with retinoid cream, imiquimod cream, and 5-flourouracil cream may be used in treatment of superficial and multiple BCCs lesions2. Treatment with oral retinoid may suppress the new BCCs lesions and result in reduction of symptoms. Recently, another therapeutic option is photodynamic therapy, involving use of photosensitizing agents that have preferentially specific effects on malignant cells3.
In conclusion, we can diagnose BCNS clinically according to 4 major criteria, which include the following: (1) discrete pigmented BCCs lesions on the face, back, and thigh proven by histology, showing several patterns, including nodular, superficial, and pigmented types of BCCs; (2) multiple palmar pits; (3) odontogenic keratocyst of the left maxillary sinus and (4) calcified falx cerebri. Since only one case of BCNS associated with various histologic patterns of BCCs has been reported in Korea, we hereby report on an interesting case of BCNS showing several histologic types of BCCs.
Figures and Tables
Fig. 1
Nodular pigmented lesion with a rolled border on the right nasolabial fold (A). Multiple, various-sized pigmented lesions on the upper back, some of which are flat, erythematous, well-circumscribed, hyperpigmented patches (B, C). Dark colored and ulcerative plaque on the right thigh (D)
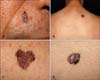
Fig. 2
Multiple pits on the palm (A). Skull X-ray shows ectopic calcification in the falx cerebri (B) and facial CT shows a well-defined round cystic mass in the left maxillary sinus with peripheral thin wall calcification (C).
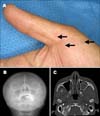
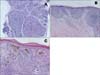
Fig. 3
Histologic findings. Nodular type (nasolabial fold); a number of round or oval tumor islands and stromal retraction within the upper to mid dermis (A). Superficial type (back); multiple buds of basophilic cells extending into the papillary dermis from the basal layer of the epidermis (B). Mixed pattern (thigh); features of superficial, nodular, and some pigmented types (C) (H&E, ×100).
References
1. Lo Muzio L, Nocini P, Bucci P, Pannone G, Consolo U, Procaccini M. Early diagnosis of nevoid basal cell carcinoma syndrome. J Am Dent Assoc. 1999. 130:669–674.

2. Yordanova I, Gospodinov D, Kirov V, Pavlova V, Radoslavova G. A familial case of Gorlin-Goltz syndrome. J IMAB-Annual Proceeding (Scientific Papers). 2007. 13:59–63.

3. Lo Muzio L. Nevoid basal cell carcinoma syndrome (Gorlin syndrome). Orphanet J Rare Dis. 2008. 3:32.

4. Kim HM, Lee CH, Kim SK, Sung TJ. Basal cell nevus syndrome (Gorlin syndrome) confirmed by PTCH mutations and deletions. Korean J Pediatr. 2007. 50:789–793.

5. Ryu DJ, Kwon YS, Roh MR, Lee MG. Two cases of nevoid basal cell carcinoma syndrome in one family. Ann Dermatol. 2008. 20:221–225.

6. Zackheim HS, Loud AV, Howell JB. Nevoid basal cell carcinoma syndrome. Some histologic observations on the cutaneous lesions. Arch Dermatol. 1966. 93:317–323.

9. Joh GY, Kim KH, Nam JT, Kim YS, Lee BK. Two cases of nevoid basal cell carcinoma syndrome. Korean J Dermatol. 1992. 30:951–957.
10. Goldstein AM, Stewart C, Bale AE, Bale SJ, Dean M. Localization of the Gene for the Nevoid Basal Cell Carcinoma Syndrome. Am J Hum Genet. 1994. 54:765–773.
11. White JC. Multiple benign cystic epitheliomas. J Cutan Genitourin Dis. 1894. 12:477–484.
12. Gorlin RJ, Goltz RW. Multiple nevoid basal-cell epithelioma, jaw cysts and bifid rib. A syndrome. N Engl J Med. 1960. 262:908–912.

13. Howell JB, Anderson DE. "The basal-cell nevus" by Howell and Caro, January 1959. Commentary: The nevoid basal cell carcinoma syndrome. Arch Dermatol. 1982. 118:813–826.

14. Evans DG, Ladusans EJ, Rimmer S, Burnell LD, Thakker N, Farndon PA. Complications of the naevoid basal cell carcinoma syndrome: results of a population based study. J Med Genet. 1993. 30:460–464.

15. Shanley S, Ratcliffe J, Hockey A, Haan E, Oley C, Ravine D, et al. Nevoid basal cell carcinoma syndrome: review of 118 affected individuals. Am J Med Genet. 1994. 50:282–290.

16. Park JW, Kim HC, Gyung MS, Shin DH, Choi JS, Kim KH, et al. A case of nevoid basal cell carcinoma syndrome. Korean J Dermatol. 2000. 38:1218–1224.
17. Tak WJ, Shin BJ, Kim MN, Ro BI. A case of nevoid basal cell carcinoma syndrome with multiple, symmetrically distributed basal cell carcinomas. Korean J Dermatol. 2002. 40:682–685.
18. Roh BH, Park SJ, Lee SY, Lee JS, Whang KU. A case of nevoid basal cell carcinoma syndrome with meningioma and sclerosing stromal tumor of the ovary. Korean J Dermatol. 2006. 44:1030–1033.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download