Abstract
Erlotinib is a small-molecule tyrosine kinase inhibitor (TKI) of the epidermal growth factor receptor (EGFR). Erlotinib has been used primarily to treat non-small cell lung cancer. In addition to its role in tumor cells, EGFR is also an important regulator of growth and differentiation in the skin and hair. Therefore, EGFR-TKIs have been associated with a number of cutaneous side effects including follicular acneiform eruptions, cutaneous xerosis, chronic paronychia, desquamation, seborrheic dermatitis, and hair texture changes. Herein, we report a rare case of a 61-year-old woman who was treated with erlotinib and experienced cicatricial alopecia.
Erlotinib is a tyrosine kinase inhibitor (TKI) that selectively inhibits the epidermal growth factor receptor (EGFR). It acts by inhibiting intracellular phosphorylation of the tyrosine kinase associated with the EGFR1. EGFR-TKIs are currently used in the treatment of a number of solid tumors. Various cutaneous side effects of EGFR-TKIs have been reported, including acneiform eruptions, chronic paronychia, xerosis, a seborrheic dermatitis-like eruption, changes in hair texture, and nonscarring alopecia1. The most common skin manifestation is a rash that has been described as a follicular or papulopustular eruption. However, significant or severe alopecia has rarely been reported in patients that receive EGFR-TKI monotherapy1,2.
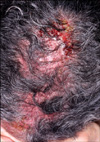
A 61-year-old woman with metastatic non-small cell lung cancer (NSCLC) presented with erythematous erosive patches containing pustules on her scalp. She had been using 150 mg/day erlotinib (Tarceva®) for 11 months. Two months after starting drug administration, she experienced a mild acneiform facial eruption. By applying a topical macrolide, it improved after several days. However, about 9 months after taking the medicine, painful, erosive patches appeared on her scalp, which was accompanied by hair loss. Physical examination showed erythematous erosive patches with follicular pustules on the scalp (Fig. 1). No other adverse events were observed, including xeroderma or inflammation of the nails. A biopsy specimen taken from the scalp showed ruptured hair follicles and abundant perifollicular infiltrate with lymphocytes and neutrophils (Fig. 2). Bacterial and fungal stains were negative, but Staphylococcus aureus was cultured from the pustules. Routine laboratory investigation found no significant abnormalities.
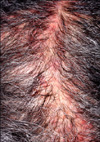
Treatment with topical and oral steroids plus antibiotics was started and continued for 5 weeks. The dosage of erlotinib was decreased from 150 mg/day to 100 mg/day, and then the erythematous, pustular skin lesion gradually improved. However, an atrophic alopecic patch developed on her scalp (Fig. 3). The final diagnosis was cicatricial alopecia, and the alopecic lesion remained unchanged during a 9-months follow-up.
EGFR inhibitors have been approved for the treatment of NSCLC, pancreatic cancer, colorectal cancer and head and neck cancer3. Targeting the EGFR pathway with a small-molecule EGFR-TKI (erlotinib) or a monoclonal antibody (cetuximab) prolonged survival in patients with advanced disease in both the first- and second-line settings4.
EGFR inhibitors can cause a range of adverse cutaneous reactions of variable severity. The most common skin toxicity is an acneiform or papulopustular rash that primarily affects the sebaceous areas of the scalp, face, and upper trunk. The rash can be itchy and, as a result, complicated by a secondary bacterial infection. The second most common skin toxicity affects the nails and includes symptoms such as discoloration, pitting, and paronychia3. Patients treated with EGFR inhibitors also sometimes exhibit hair abnormalities, like excessive eyelash and eyebrow growth or curly/wavy hair on the face or scalp that is both fine and brittle2,3. Substantial alopecia is uncommon. A literature search identified five other cases of alopecia associated with EGFR inhibitors; but, only one case of cicatricial alopecia was reported (Table 1)1,2,5-7.
The mechanism underlying the folliculocentric rash remains unclear, although it is known that EGFR inhibitors can have several adverse effects on epithelial homeostasis. EGFR is strongly expressed in the basal layer of the epidermis, with lower expression in the lower dermal papilla, outer root sheath of the hair follicle, outer sheath of the upper hair shaft, sebaceous glands, and eccrine sweat glands. Inhibiton of these EGFRs leads to growth and migratory abnormalities that result in a papulopustular rash and impaired differentiation3,8.
Several studies have shown that EGFRs play an essential role in the maintenance of normal hair follicles. In 2002, Kimyai-Asadi and Jih9 reported that a chimeric anti-EGFR antibody was toxic to follicles. EGFR-knockout mice had thin skin with poorly defined stratification and altered terminal differentiation of the epidermis and hair follicles. Failure of hair to enter the catagen stage resulted in a severe inflammatory reaction in the surrounding skin, follicular necrosis, and alopecia. In addition to its essential role in hair cycle regulation, EGFR also plays a role in regulating inflammation. This could be important to the pathogenesis of inflammatory infiltration and the destruction of hair follicles6. Based on these observations, the folliculocentric pustular rash was not considered to be the cause of infection; moreover, this hypothesis was supported by results obtained in microbiological cultures. The folliculocentric puspular rash is thought to result from abnormal keratinization, follicular retention and subsequent rupture of the affected hair follicle10. The present case exhibited erosive patches and pustules on the scalp, and S. aureus was cultured form the pustules. Histological findings showed folliculitis with an infiltrate of mixed inflammatory cells. We postulated that these findings were likely due to a secondary infection that resulted from abnormal keratinization of the hair follicles and a failure to control the inflammatory process due to EGFR inhibition.
During most of the hair cycle, the lower portion of the hair follicle is an immune-privileged site6. However, during follicle regression in the catagen stage, major histocompatibility complex class 1 antigens are expressed in the lower portion of the follicle, then activated macrophages infiltrate the area and the lower portion of the follicle degenerates6. EGFR induces suppression of free radical production, which might be necessary to control the inflammation process. Without this EGFR function, the expression of major histocompatibility complex class 1 antigens in the early catagen stage and the following inflammatory response could lead to the destruction of the hair follicle6. Thus, it is possible that cicatricial alopecia resulted from immune privilege failure in the hair follicle. Alternatively, development of cicatricial alopecia may result from various stimuli that induce the inflammatory infiltrate11. Possible antigenic stimuli include ultraviolet light in patients with discoid lupus erythematous in the scalp, certain medications in patients with lichen planopilaris, and S. aureus infection in patients with folliculitis decalvans11. In the case presented here, S. aureus may have aggravated the course of EGFR-induced cicatricial alopecia development.
Cicatricial alopecia caused by an EGFR inhibitor is relatively rare. Generally no bacteria are associated with follicular inflammation induced by treatment with an EGFR inhibitor. However, S. aureus was identified in this case. Histological findings indicated that S. aureus might have continued to break up follicular units and thus, aggravated inflammation. We hypothesized that these conditions might have caused cicatrical alopecia; however, accurate identification of the underlying mechanism requires further study.
Figures and Tables
References
1. Pongpudpunth M, Demierre MF, Goldberg LJ. A case report of inflammatory nonscarring alopecia associated with the epidermal growth factor receptor inhibitor erlotinib. J Cutan Pathol. 2009. 36:1303–1307.

2. Costa DB, Kobayashi S, Schumer ST. Erlotinib-associated alopecia in a lung cancer patient. J Thorac Oncol. 2007. 2:1136–1138.

3. Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors. Target Oncol. 2009. 4:107–119.

4. Langer C, Soria JC. The role of anti-epidermal growth factor receptor and anti-vascular endothelial growth factor therapies in the treatment of non-small-cell lung cancer. Clin Lung Cancer. 2010. 11:82–90.

5. Donovan JC, Ghazarian DM, Shaw JC. Scarring alopecia Vol. 23, Suppl. 3, 2011 S353 associated with use of the epidermal growth factor receptor inhibitor gefitinib. Arch Dermatol. 2008. 144:1524–1525.
6. Graves JE, Jones BF, Lind AC, Heffernan MP. Nonscarring inflammatory alopecia associated with the epidermal growth factor receptor inhibitor gefitinib. J Am Acad Dermatol. 2006. 55:349–353.

7. Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005. 6:491–500.

8. Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006. 55:657–670.

9. Kimyai-Asadi A, Jih MH. Follicular toxic effects of chimeric anti-epidermal growth factor receptor antibody cetuximab used to treat human solid tumors. Arch Dermatol. 2002. 138:129–131.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download