Abstract
Vandetanib is a once-daily oral anticancer drug that selectively inhibits key signaling pathways in cancer by targeting vascular endothelial growth factor receptors, epidermal growth factor receptors tyrosine kinase, and rearranged during transfection-dependent tumor cell proliferation and survival. The most frequently reported adverse events attributed to vandetanib include diarrhea, elevated aminotransferase, asymptomatic corrected QT interval prolongation, and hypertension. Though a number of randomized, doubleblind studies, including cutaneous adverse events attributed to vandetanib, have been reported along with these general symptoms, no case of Stevens-Johnson syndrome (SJS) has been reported. This paper demonstrates a case of SJS induced by vandetanib.
Vandetanib (Zactima®, AstraZeneca Pharmaceuticals, London, UK) is an oral anticancer drug, used alone or combined with doxetacel that has demonstrated a significant prolongation of progression-free survival in non-small-cell lung carcinoma (NSCLC). Although Phase I clinical evaluation in patients with solid tumors showed that vandetanib monotherapy (up to 300 mg/d) was generally well tolerated, concerns have been expressed over the safety of vandetanib1. Based on randomized Phase II clinical trials, the common vandetanib-related toxicities have been known to be as diverse as from dyspnea, possibly related to comorbid illness, to "rash"2,3. These cutaneous events have been reported to have mild to moderate severity; a few patients developed skin eruptions of toxicity grade 32. Currently, no case of life-threatening cutaneous eruption such as Stevens-Johnson syndrome (SJS) induced by vandetanib has been reported domestically or overseas.
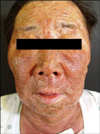
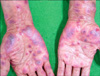
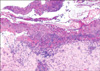
A 71-year old man was referred for consultation of diffuse erythematous targetoid maculopatches mainly on the face, neck and upper extremities. The patient had a history of non-small-cell lung carcinoma from July 2009. He had been treated with vandetanib at a dose of 300 mg daily. On day 21 of vandetanib treatment, he presented with multiple erythematous maculopatches, mainly on the face and neck. Five days later he exhibited multiple purpuric coalescing macules and vesicles on face and neck (Fig. 1). Diffuse erosion of oral mucosa and purpuric macules with flat atypical targets were observed on the face, palms (Fig. 2), soles, and trunk. Epidermal detachment developed approximately above 15 percent of the patient's body surface area. He had a body temperature of 38.3℃, and complained of odynophagia and conjunctivitis. Routine laboratory examinations revealed leukocytosis (17,500/mm3, NL (normal limit): 3,600~10,200/mm3), elevated eosinophil levels (11.4 IU/L, NL: 0~8.2 IU/L), and abnormal alanine aminotransferase (ALT, 53 IU/L, NL: 0~41 IU/L). Bacterial cultures from blood, urine, and sputum revealed no evidence of bacterial infection. Histopathologic findings of biopsy on the patient's right forearm showed diffuse epidermal necrosis, including necrotic keratinocytes, eosinophils, and severe perivacular infiltration of lymphocytes (Fig. 3). Considering that overall lesions showed violaceous targetoid macules, vesicular lesions diffusely developed on his whole body including extremities, palms, soles, trunk, oral mucosa, and conjunctiva, and skin biopsy showed epidermal necrosis and detachment, these lesions were diagnosed as vandetanib-induced SJS. We recommended his oncologist to discontinue the administration of the drug immediately. The skin lesions gradually resolved for 15 days after withdrawal of vandetanib, followed by antihistamines, topical corticosteroid, and wet dressing. The patch or provocation test could not be performed for safety reasons though it was needed for confirmation of the diagnosis. However, the patient had no history of taking any medication other than vandetanib, and no history of recurrent herpes simplex virus, mycoplasma pneumonia or other infections which were firmly established as the major causes of erythema multiforme. Considering that no proof of bacterial infection was observed in his blood, urine and sputum, we came to the conclusion that vandetanib had played a decisive role in his illness. There was no recurrence when he was followed up in three months.
SJS is an acute life-threatening muco-cutaneous disease characterized by extensive necrosis and detachment of the epidermis4. According to Bastuji-Garin et al., consensus classification was proposed as: bullous erythema multiforme, detachment below 10 percent of the body surface area plus localized "typical targets" or "raised atypical targets"; SJS, detachment above 10 percent of the body surface area plus widespread erythematous or purpuric macules or flat atypical targets5. The overall incidence of SJS was estimated at about one or two cases per million person-years4. More than 100 different drugs have been reported as possible causes of SJS, including primarily allopurinol, sulfonamides, barbiturates, lamotrigine, phenytoin, oxicam non-steroidal anti-inflammatory agents (NSAIDs), and aminopenicillin4. The precise sequence of molecular and cellular events of SJS is not completely understood. However, the immunologic pattern of early lesions suggests a cell mediated cytotoxic reaction against keratinocytes, leading to massive apoptosis4.
Along with common adverse effects such as headache, nausea, diarrhea, constipation, abdominal distention/bloating, elevated aspartate transaminase/alanine transaminase and hypomagnesemia, the common cutaneous adverse events attributed to vandetanib have been reported as acneiform rash, photosensitivity, alopecia and hand-foot syndrome6. Horti et al. reported common adverse events that were related to vandetanib, including erythematous rash (14 percent), and severe exfoliative rash (12 percent) in their randomized, controlled study2. According to another randomized double-blind controlled study, patients developed grade three skin desquamation, which meant a severe or medically significant condition requiring hospitalization, but not immediately life-threatening. Recent clinical trials revealed that vandetanib has been linked to serious adverse events higher than grade three in approximately 55 percent of patients who used the medication7. The Food and Drug Administration Briefing Document Oncologic Drugs Advisory Committee Meeting in December, 2010 reported severe adverse events of vandetanib and is now asking an expert panel to discuss the medication and determine whether the "substantial toxicity" of vandetanib means that the drug should have limited indications8. This toxicity includes ischemic arterial events, interstitial lung disease, and SJS. SJS and toxic epidermal necrolysis were reported in 0.7 percent of cases according to "significant adverse events" in the vandetanib safety database8. Risk factors for evolution of rash into SJS are unclear, with eight of 21 patients receiving radiation prior to development of SJS8.
For SJS, Mockenhaupt et al.9 classified marketed drugs as "highly suspected" drugs, namely anti-infective sulfonamides, anticonvulsants, oxicam-NSAIDs, and nonnucleoside anti-retroviral agent, the anticonvulsant lamotrigine, "significant but lower risk" drugs such as NSAIDs, macrolides, and cephalosporins, "non-significant risk" drugs such as fluoxetine, other serotonin reuptake inhibitors, proton pump inhibitors, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, and "not assessable" drugs such as terbinafine, fluconazole, and cyclooxygenase 2 inhibitors. As Vandetanib is still an experimental drug, it has not been classified or verified in view of risk of SJS. Though clinical trials have revealed substantial risk posed by vandetanib, SJS attributed to this drug has not been presented as a case report or specifically evaluated in sufficient numbers of randomized controlled studies.
Physicians ought to consider the possibilities of drug hypersensitivity such as SJS as seen in our case, often leading to fatal outcomes, though the safety or tolerability of vandetanib has not been established. Clinical trials on vandetanib are currently underway upon the effectiveness of treating a variety of tumors, safe dosage, and adverse events attributed to it. Though vandetanib-induced drug eruption has not been frequently reported as of yet, the occurrence of adverse events attributed to the drug as shown above suggests discreet prescription of vandetanib and setting up of mandatory education of cutaneous adverse events induced by the drug would be beneficial.
Figures and Tables
References
1. Heymach JV. ZD6474--clinical experience to date. Br J Cancer. 2005. 92:Suppl 1. S14–S20.
2. Horti J, Widmark A, Stenzl A, Federico MH, Abratt RP, Sanders N, et al. A randomized, double-blind, placebocontrolled phase II study of vandetanib plus docetaxel/prednisolone in patients with hormone-refractory prostate cancer. Cancer Biother Radiopharm. 2009. 24:175–180.

3. Wells SA Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010. 28:767–772.

4. Auquier-Dunant A, Mockenhaupt M, Naldi L, Correia O, Schröder W, Roujeau JC. SCAR Study Group. Severe Cutaneous Adverse Reactions. Correlations between clinical patterns and causes of erythema multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis: results of an international prospective study. Arch Dermatol. 2002. 138:1019–1024.
5. Bastuji-Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme. Arch Dermatol. 1993. 129:92–96.

6. Annunziata CM, Walker AJ, Minasian L, Yu M, Kotz H, Wood BJ, et al. Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2. Clin Cancer Res. 2010. 16:664–672.

7. AstraZeneca core presentation: Vandetanib for unresectable locally advanced or metastatic MTC, Antoine Yver. accessed January 2011. www.fda.gov.
8. FDA Briefing Document Oncologic Drugs Advisory Committee Meeting. 2010. 12. 02. accessed December 2010. NDA 22405/S000 Vandetanib www.fda.gov.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download