INTRODUCTION
Aesthetic augmentation of the penis has generated increasing interest among men. Most men who request surgical penile enhancement have a normal-sized and fully functional penis but perceive their penises to be small. This feeling can be exacerbated by advertising in the media1. There are no recommended indications for penile girth enhancement procedures in the medical literature, and no guidelines have been proposed for this technique2. The injection of exogenous substances, such as autologous fat, silicone, hyaluronic acid gel, and dermal fat into the genital skin to cause penile enlargement occurs in many cultures3. BellaGen™ (Hans Biomed, Seoul, Korea) is an injectable, inert, acellular dermal matrix that is intended for the repair or replacement of damaged soft tissue. Recently, SureDerm™ (Hans Biomed, Seoul, Korea), a pro-powdered state acellular dermal matrix, has been used as an alternative to autologous fat injections or dermal fat grafts for penile girth enhancement4. As BellaGen™ injections are more convenient and faster than SureDerm™, many local clinics use this product for soft tissue augmentation. Complications from BellaGen™-based phalloplasties have not been described in the literature. We report here on an inflammatory reaction and necrosis secondary to the injection of BellaGen™ for the enhancement of penile girth.
CASE REPORT
A 50-year-old Korean male presented to our department with a painful ulcer on the dorsal side of the penis. He had received injections of BellaGen™ in the penis for penile girth enhancement at a private urology clinic 2 weeks prior. Three days after the injections, erythema and intermittent swelling were noted on the dorsal side of the penis, and an erosive, severely painful lesion subsequently developed. Conservative wound management had been prescribed, but the patient reported little improvement.
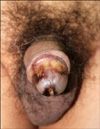
On exam, there was an erosive lesion with necrotic changes of the glans penis and the dorsal side of the penis (Fig. 1). Otherwise, the remainder of the physical examination was unremarkable. The patient had a 15 year history of type II diabetes mellitus with poor glucose control. Penile and scrotal magnetic resonance imaging was normal. A biopsy was performed with a working diagnosis of foreign body reaction.
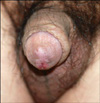
The histopathological examination showed a moderate lymphohistiocytic infiltration with necrotic changes (Fig. 2). The patient was treated with conservative wound management--wet dressings with KMNO4 solution, occlusion with gauze of petroleum jelly, and a 4-week course of oral antibiotics. During treatment, he developed complete skin necrosis of the glans penis. He underwent aggressive debridement of the necrotic penile skin, and conservative wound care was continued. After 8 weeks, the lesion improved, and his pain gradually decreased (Fig. 3).
DISCUSSION
Acellular inert dermal matrices derived from donated human skin tissue have been sporadically used for soft tissue augmentation in many medical departments5-7. Recently, with increasing numbers of patients paying attention to aesthetics and cosmetics, acellular dermal matrices have even been used for penile girth procedures. The use of AlloDerm for penile girth enhancement has the potential to provide a more consistent cosmetic result with respect to symmetry and durability. It is also associated with a lower relative incidence of adverse effects when compared to dermal fat grafting3.
Zhang et al.8 reported that penile enlargement by implantation of multiple layers of acellular dermal matrix was a safe and effective operation. SureDerm™ is a sheet-type of AlloDerm made in Korea. These sheets have been placed above the superficial lamina of Buck's fascia at the interface with the dartos fascia to enhance penile and granular girth3. The advantages of this technique are many: no rejection response, no donor-site scarring, good graft survival, and a low complication rate4. Despite its many advantages, it still requires a fairly complex procedure and insertion time. To make the product less time consuming, it was made into a powdered form and commercialized as BellaGen™. BellaGen™ is an injectable form of an acellular dermal matrix that is easy to insert and requires less injection time, garnering a favorable reception by office workers since its introduction.
Despite the many advantages of acellular dermal matrices, when used to enhance penile girth in a cosmetic phalloplasty, the viability of the overlying thin and delicate penile skin can be compromised. Other complications associated with the use of acellular dermal matrices include erosion, fibrosis, infection, resorption, and skin loss, which can have a detrimental effect on penile length and function9. Due to limited data, the use of acellular dermal matrices in penile girth enhancements must be closely scrutinized.
In our presented case, necrosis of the glans and dorsal penile skin occurred after BellaGen™ injection, creating a unique and difficult problem to manage. There are several explanations for why this complication may have occurred. The cause of the skin necrosis was likely from disruption of the underlying penile vasculature. The arterial blood supply to the penile shaft and prepuce is provided by the bilateral superficial penile arteries, which branch from the inferior external pudendal arteries9. Our patient had a 15-year history of diabetes with poor glucose control. High blood sugar levels can damage nerves and contribute to blood vessel narrowing, thereby disrupting the nerve and blood supply to the penis. In addition to poorly controlled diabetes, the BellaGen™, though theoretically providing increased cosmetic girth, was an avascular component introduced into the tissues and may have inhibited the onset of inosculation, ultimately hindering skin viability10. The location of the injection is also important to note. Unlike acellular dermal matrix sheets, injections can more easily be misplaced due to variations in needle depth or an operator's mistake. Dermal matrix should be placed above the superficial lamina of Buck's fascia at the interface with the dartos fascia3, but if the dermal matrix were to reach the carvernosum of the penis, it could cause necrosis. Though rare, dermal matrix could also cause an allergic reaction. Acellular dermal matrix, like hyaluronic acid, has no organ or species specificity and theoretically poses no risk of allergy. However, Micheels11 has reported the presence of circulating antibodies against hyaluronic acid in patients, which supports the allergy hypothesis given that it is possible for circulating antibodies against dermal matrix to exist. Our patient experienced inflammatory reactions after the intradermal injections, complaining of severe redness, swelling, pain, and tenderness, which may have been allergic reactions.
We treated our patient with conservative wound management, oral antibiotics, and aggressive debridement of necrotic skin, which improved the condition of the lesion. However, he looked behind distressing days after procedures.
This case demonstrated an inflammatory and necrotic reaction to locally injected BellaGen™. Though rare, acellular dermal matrix injections for cosmetic purposes may produce severe side effects. With the increasing availability of diverse soft tissue augmentation materials, physicians should be aware of this complication from injectable acellular dermal matrix and carefully consider whether patients interested in receiving injections may have any diseases that would influence blood supply and tissue viability.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download