Abstract
Herein, we report a case of an 8-year-old girl with dapsone hypersensitivity syndrome (DHS) that occurred during the treatment of erythema elevatum diutinum. She had fever, gross hematuria, and malaise for three weeks after initiation of dapsone therapy. Five days after stopping dapsone treatment, she returned to the emergency clinic because of high fever, emesis, diarrhea, upper respiratory symptoms, and worsening of exanthematous eruptions. A diagnosis of DHS was made, and it improved with oral prednisone. We recommend that pediatric patients who are treated with dapsone need to be observed carefully for the development of DHS.
Dapsone is indicated for the treatment of leprosy, but it is also used for various skin diseases1. Moreover, dapsone is the drug of choice for the management of erythema elevatum diutinum (EED)2. The most frequent associated side effects are dose-related hemolytic anemia and methaemoglobinemia2. More rarely, dapsone can cause severe adverse effects, such as dapsone hypersensitivity syndrome (DHS) or agranulocytosis1. DHS typically starts within eight weeks of initiating dapsone treatments and is characterized by fever, rash, hemolytic anemia, lymphocytosis and hepatitis1,3. Herein, we report DHS that occurred during the treatment of a pediatric patient with EED.
An 8-year-old girl presented with tender papules and nodules on the extensor surfaces of the extremities that had been there for seven months. A physical examination revealed firm, erythematous to skin-colored papules and nodules on her both hands, wrist, feet, elbows, and knees (Fig. 1). The histopathologic examinations of the skin lesions from her hand revealed widespread vasculitis in the small vessels of the dermis with fibrinoid deposits and extravasated red blood cells. The infiltrates were composed of multiple small aggregates of histiocytes, neutrophils, and nuclear fragments (Fig. 2). Thus, the clinical and histopathological findings were consistent with a diagnosis of EED.
Despite several months of potent topical and systemic steroid therapy, the cutaneous lesions remained, and she was started on dapsone treatment. The dosing regimen of dapsone consisted of taking 100 mg daily for two days and skipping for one day. A dramatic and rapid response was seen within two weeks of initiation of dapsone therapy.
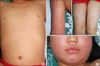
However, she stopped the dapsone treatment after three weeks of treatment due to gross hematuria, malaise, fever, and cough. At that time, she was thought to have a viral illness or an unrelated upper respiratory infection. Nevertheless, five days after stopping treatment of dapsone, she returned to the emergency clinic because of high fever, emesis, diarrhea, upper respiratory symptoms, and a worsening rash. She also had maculopapular exanthematous eruptions with facial edema and lymphadenopathies (Fig. 3). She was hospitalized, and blood samples were taken for routine examination, including viral serology, bacterial culture, complement levels, and autoimmune screening.
A complete blood count revealed a hemoglobin of 9.7 mg/dl, a hematocrit of 31.0, a white blood cell count of 30,110/mm3, reticulocyte count of 5.74%, a platelet count of 124,000/mm3, and a C-reactive protein of 0.51 mg/dl in the first hour. Her liver function tests were abnormal: aspartate aminotransferase 441 U/L, alanine aminotransferase 657 U/L, alkaline phosphatase 1,023 U/L, total bilirubin 6.06 mg/L, direct bilirubin 3.88 mg/dl, prothrombin time 16.2 seconds, international normalized ratio 1.35, partial thromboplastin time 43.0 seconds, and lactate dehydrogenase 2,221 U/L. Titers were negative for viral hepatitis serology (hepatitis A, B, and C) and Epstein-Barr virus. Although there was gross hematuria, the levels for urea, creatinine, and electrolyte in the blood were within normal limits. Bacterial cultures (blood and urine), levels of complement (C3, C4, and CH50), and autoimmune screen (antinuclear antibody) were all negative or within normal limits.
A diagnosis of DHS was made, and the patient was treated with oral prednisone (60 mg/day). Her condition improved quickly and laboratory test results returned to normal levels within two weeks.
EED is a chronic recurrent form of cutaneous leukocytoclastic vasculitis thought to be immune-complex mediated4,5. It typically presents as multiple, persistent, symmetric and erythematous to violaceous papules/plaques on the extensor surfaces of the extremities4,5. The histopathologic features characteristic of EED are not usually all present within the same lesion5. A spectrum from leukocytoclastic vasculitis to vessel occlusion and dermal fibrosis are observed5. Early stage lesions are characterized by neutrophilic, perivascular infiltrates with dermal fibrin deposits, endothelial expansion, and leukocytoclasis5,6. With disease progression, a granulation tissue-like response with dermal fibrosis and capillary proliferation become the predominant features5,6. Diagnosis of EED must be based on a characteristic clinical presentation and confirmatory histopathological findings5,6.
Dapsone has been broadly used for treatment of leprosy and a wide variety of dermatological inflammatory diseases because of its excellent anti-inflammatory and immunomodulatory effects1. Generally, dapsone or sulfonamides are considered to be a first-line treatment for EED5,6.
The responsiveness of EED to dapsone is thought to be secondary to its inhibitory effects on neutrophil chemotaxis and function2. Therefore, we tried dapsone therapy in our patient, because her lesions did not respond to topical and systemic steroids. Although a dramatic and rapid response was seen within two weeks of initiation of therapy, DHS occurred three weeks after initiation of dapsone therapy.
The most frequent side effects of dapsones are doserelated methemoglobinemia and hemolytic anemia, and rarely, it can cause an idiosyncratic reaction, called dapsone hypersensitivity syndrome1-3. DHS has been reported for a variety of dermatological conditions, including leprosy, dermatitis herpetiformis, acne vulgaris, psoriasis, leukocytoclastic vasculitis, cicatrical pemphigoid, pemphigus and lupus erythematosus1,3.
A true diagnosis of DHS should be made based on the following criteria: 1) symptoms manifesting within eight weeks of starting therapy and resolving after withdrawal of the drug, 2) symptoms not attributable to any other drug used simultaneously, and 3) symptoms unrelated to leprosy or any underlying disease7,8.
DHS is a severe, multiorgan reaction to dapsone that includes fever, rash, jaundice, lymphadenopathy, splenomegaly and pedal edema1,3. Hemolytic anemia, atypical lymphocytosis and hepatitis are other accompanying findings1,3. Of note, fever almost invariably presents as the initial sign9. In addition, prominent edema of the face, particularly in the periorbital area, is a noticeable feature of drug induced hypersensitivity syndrome9. The rash, which is often initially a benign morbilliform eruption, may develop into exfoliative dermatitis1,3.
Our patient showed consistent clinical and laboratory findings of DHS and her symptoms appeared within three weeks of starting on dapsone. In particular, it is noteworthy that hemolytic anemia was also present, as proved by decreased hemoglobin, high reticulocyte count, and high bilirubin. In addition, it is essential to check levels of enzyme glucose 6-phosphate hydrogenase (G6PD) before beginning dapsone, because G6PD-deficient patients may experience severe hemolysis1-3.
It is presumed in the pathogenesis of DHS that hypersensitivity to dapsone may be caused by metabolites of dapsone-forming haptens with the formation of anti-dapsone antibodies10,11. The exact incidence of DHS is not known, but it is reported to occur in less than 1% of patients treated with dapsone1,3. According to a study by Agrawal and Agarwalla3, the mean age in patients with DHS was 33.2 years (range 13 to 64 years). Although aging was a relatively adverse predisposing factor for side effects of daponse1, there was no higher predominance of DHS in pediatric patients. Most of the patients were below 50 years of age and this may be because of the decreased enzyme activity and production of toxic metabolites with aging1.
Typically the symptoms of DHS begin within two to six weeks of the start of therapy (Table 1). However, it can appear as early as six hours in a previously sensitized individual or as late as six months after the start of dapsone therapy12. Due to its significant enterohepatic circulation, dapsone has a long elimination half-life that averages between 24 and 30 hours13. This is important to remember in case adverse reactions emerge after a long metabolite impact period14. The initial dosage is crucial in DHS, and Labandeira and Toribio15 suggest that a high dose (>50 mg/day) in the first six to eight weeks of treatment is advisable in patients who do not have leprosy. A mortality rate of 11~13% has been reported, and hepatic encephalopathy is prominent in fatal cases16.
DHS is generally a self-limiting drug reaction, and most patients recover following cessation of dapsone therapy14. However, a systemic corticosteroid is frequently used in its treatment. The duration of recovery has been shown to be shorter in patients who received a systemic corticosteroid, although no controlled studies have been performed to evaluate it effectiveness1,3.
To our knowledge, there has been one reported case of DHS occurring in patients with EED. Potter et al.17 reported the case of a 68-year-old man with EED who had been taking dapsone, 200 mg/day, and developed DHS three weeks after commencement of the drug. In fact, it is unclear whether EED itself affects DHS, because this case is the second case of DHS during treatment of EED. However, the use of dapsone is recently decreasing in dermatologic inflammatory disease because of the reduction of leprosy patients and development of new drugs. Moreover, it is difficult to find any dermatologic diseases for which dapsone is recommended as first line treatment. In this regard, it is highly suggestive that dapsone or sulfonamides are still considered a first-line treatment for EED. Since treatment of EED remains challenging due to the chronic and recurrent nature of the disease, dapsone was necessary to use in our patient for the treatment of EED.
In conclusion, we emphasize that more attention is needed for dapsone therapy in the patient with EED. In addition, physicians, especially those prescribing drugs for pediatric patients, should be alert to this rare but potentially life-threatening adverse drug effect.
Figures and Tables
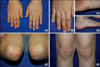 | Fig. 1(A) Multiple, persistent, symmetric and erythematous to skin-colored papules and nodules on the extensor surfaces of the hands. (B) Close up view of the right hand, (C) right wrist, (D) right foot, (E) elbows and (F) knees. |
 | Fig. 2(A) Biopsy of an early lesion from the hand revealed widespread vasculitis in the small vessels of the dermis with fibrinoid deposits and extravasated red blood cells (H&E, ×100). (B) The infiltrates were composed of multiple small aggregates of histiocytes, neutrophils, and nuclear fragments (H&E, ×400). |
References
2. Sago JG, Hall RP. Wolff K, Goldsmith LA, Katz SI, Gilehrest BA, Paller AS, Leffell DJ, editors. Dapsone. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;2154–2157.
3. Agrawal S, Agarwalla A. Dapsone hypersensitivity syndrome: a clinico-epidemiological review. J Dermatol. 2005. 32:883–889.

4. Wahl CE, Bouldin MB, Gibson LE. Erythema elevatum diutinum: clinical, histopathologic, and immunohistochemical characteristics of six patients. Am J Dermatopathol. 2005. 27:397–400.
5. Comfere NI, Gibson LE. Wolff K, Goldsmith LA, Katz SI, Gilehrest BA, Paller AS, Leffell DJ, editors. Erythema elevatum diutinum. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;1616–1619.
7. Richardus JH, Smith TC. Increased incidence in leprosy of hypersensitivity reactions to dapsone after introduction of multidrug therapy. Lepr Rev. 1989. 60:267–273.

8. Sener O, Doganci L, Safali M, Besirbellioglu B, Bulucu F, Pahsa A. Severe dapsone hypersensitivity syndrome. J Investig Allergol Clin Immunol. 2006. 16:268–270.
9. Carroll MC, Yueng-Yue KA, Esterly NB, Drolet BA. Drug-induced hypersensitivity syndrome in pediatric patients. Pediatrics. 2001. 108:485–492.

10. Uetrecht JP. Idiosyncratic drug reactions: possible role of reactive metabolites generated by leukocytes. Pharm Res. 1989. 6:265–273.
11. Rieder MJ, Uetrecht J, Shear NH, Cannon M, Miller M, Spielberg SP. Diagnosis of sulfonamide hypersensitivity reactions by in-vitro "rechallenge" with hydroxylamine metabolites. Ann Intern Med. 1989. 110:286–289.

12. Kumar RH, Kumar MV, Thappa DM. Dapsone syndrome--a five year retrospective analysis. Indian J Lepr. 1998. 70:271–276.
13. Zuidema J, Hilbers-Modderman ES, Merkus FW. Clinical pharmacokinetics of dapsone. Clin Pharmacokinet. 1986. 11:299–315.

14. Sener O, Doganci L, Safali M, Besirbellioglu B, Bulucu F, Pahsa A. Severe dapsone hypersensitivity syndrome. J Investig Allergol Clin Immunol. 2006. 16:268–270.
15. Labandeira J, Toribio J. Reinstatement of dapsone following hypersensitivity. Acta Derm Venereol. 2003. 83:314–315.

16. Sheen YS, Chu CY, Wang SH, Tsai TF. Dapsone hypersensitivity syndrome in non-leprosy patients: a retrospective study of its incidence in a tertiary referral center in Taiwan. J Dermatolog Treat. 2009. 20:340–343.

17. Potter B, Szymanski F, Fretzin D. Erythema elevatum diutinum and sulfone hypersensitivity-Society transactions. Arch Dermatol. 1967. 95:436–440.
18. Choi HW, Song IK, Chung EA, Cha DY, Lim MK, Na DJ, et al. A case of Sulfone syndrome hypersensitivity associated with dapsone. J Asthma Allergy Clin Immunol. 2001. 21:1206–1210.
19. Kim JW, Kim JS. Two cases of dapsone syndrome. Korean J Dermatol. 2005. 43:655–659.
20. Lim CH, Bae SH, Shin JA, Uhm JS, You CR, Choi JY, et al. A case of severe hepatitis caused by dapsone syndrome successfully treated with steroids. Korean J Med. 2007. 73:915–919.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download