Abstract
Although more than 90% of melanomas have cutaneous origins, melanomas sometimes present metastatically with no apparent primary lesion. A 62-year-old female presented with black pigmentation on her left thumbnail that had begun 2 years earlier and after the biopsy, she was diagnosed with malignant melanoma. Interestingly, 7 years earlier, a 4 cm palpable mass on her left axilla had been diagnosed as melanoma from an unknown primary site (MUP) with the involvement of an axillary lymph node. We speculate that the melanoma of the left thumb was the primary site and the melanoma in the axilla was a metastasis from the left thumb, and suggest several hypotheses explaining the appearance of the primary lesion as acral lentiginous melanoma after detecting a metastatic site. We consider this case interesting because it helps us to understand the pathogenesis of MUP and reminds physicians to conduct careful periodical work-ups of melanoma patients, and highlights the importance of continued long-term follow-up, especially for patients with MUP.
Melanoma occasionally occurs as apparent metastasis to lymph nodes or viscera without a detectable or known primary lesion, constituting the so-called melanoma from an unknown primary site (MUP). MUPs are estimated to comprise 3.7~6.0% of all incidental melanomas1. The clinical behavior and etiology of these lesions are poorly understood. We speculate that the melanoma of the left thumb was the primary site and the melanoma in the axilla was a metastasis from the left thumb and suggest several hypotheses explaining the appearance of the primary lesion as acral lentiginous melanoma (ALM) after detecting a metastatic site.
A 62-year-old female visited the Dermatology Department with spreading black pigmentation on her left thumbnail and brown macules on the periungual skin that had begun 2 years earlier (Fig. 1). She underwent punch biopsies, which showed many hyperplastic atypical melanocytes with dermal invasion (Fig. 2A). The atypical melanocytes stained positively for both HMB-45 and S-100 protein (Fig. 2B, C). We diagnosed the lesion as malignant melanoma, acral lentiginous type, and performed an evaluation that included computed tomography (CT), magnetic resonance imaging (MRI), and position emission tomography-computed tomography (PET-CT) to identify metastatic sites. However, no metastatic site was found and she underwent only left thumb amputation at the level of the interphalangeal joint.
Interestingly, 7 years earlier, she had visited the Department of Internal Medicine with a 4-cm palpable mass in the left axilla. On excision, it showed a histologically effaced nodal architecture involving wildly pleomorphic epithelioid cells with bizarre nuclei and variable amounts of dusty cytoplasmic pigment that stained with S100 and HMB-45, consistent with metastatic melanoma (Fig. 3). There was no family history of melanoma and she described her sun exposure as little to none. There was no history of previously excised pigmented cutaneous lesions. All the initial routine blood tests and chest x-rays were unremarkable. She underwent a full work-up, including CT, MRI, and PET-CT, to identify the primary site of the melanoma and underwent urogenital, otolaryngologic, ophthalmologic, and other pertinent examinations to exclude unusual primary sites. However, no primary site had been found and she was diagnosed with MUP at that time.
She was so concerned about this left axillary melanoma that she felt as though something was in her left breast. Breast examination including mammography, MRI, and CT proved to be all normal but she wanted to widely remove her left breast. Thus she underwent a left-sided modified radical mastectomy with level III axillary lymph node dissection after thorough explanation of sequale-like lymphedema.
Three months later, she began to develop secondary lymphedema of the left arm. Following conservative treatment with compression bandages, the lymphedema improved slowly.
Although more than 90% of melanomas have cutaneous origins, melanomas may sometimes present metastatically in the absence of a primary lesion. The natural history of metastatic melanoma involving lymph nodes, in the absence of a known primary site (cutaneous, ocular, or mucosal) is unclear; consequently, the optimal management of this rare subtype of disease also remains unclear. MUP is estimated to comprise 3.7~6.0% of all melanomas1.
We suggest several hypotheses explaining the appearance of the primary lesion as ALM after detecting a metastatic site (Fig. 4).
The first hypothesis is that a spontaneously regressed primary melanoma near the left thumbnail develops nodal metastasis and eventually recurs locally at the left thumbnail as a present illness. Regression is frequent in the natural history of melanoma. Melanomas undergo regression six times more often than do other malignant neoplasms2. Features of partial regression of a primary cutaneous melanoma are observed in 10~35% of cases3. Total regression is much less common, but numerous cases have been reported in which the primary tumor underwent complete regression after the development of nodal and distant metastases. Therefore, the primary lesion was not found clinically because it had undergone regression, which was complete at the time of diagnosis of the metastatic disease. Subsequently, the primary lesion appears clinically when locally recurrent melanoma develops. Mahrle et al.4 reported a patient with malignant melanoma that underwent spontaneous regression with simultaneous development of a secondary tumor. The author's assertion that "…regression of a melanoma may not prevent progression of the disease or formation of a new primary melanoma…" supports our hypothesis.
The primary site may have a slow growth rate; therefore, our second hypothesis is that the primary site rarely becomes manifest during the clinical course of the disease especially when the tumor shows an amelanotic picture. Recent evidence suggests that melanomas constitute a family of different tumors with varying abilities to grow and metastasize5. ALM is characterized by slow lentiginous radial growth and evolves slowly over many years6. In our case, a slow-growing AML may explain why the primary site appeared after detecting a metastatic lesion.
The third hypothesis is that an amelanotic melanoma can be detected as the primary site after lymph node metastasis. The lack of pigmentation causes the clinical appearance to be nonspecific for melanoma, so that it is difficult to find clinically. Subsequently, the amelanotic melanoma may gradually develop a pigmented lesion, which can be detected clinically. Karine reported that amelanotic lesions in albino mice developed foci of dark pigmentation7. He suggested that late events occurring within the amelanotic melanoma generate some tyrosinase activity and the production of melanin.
The last hypothesis is that the primary site of the metastatic axillary melanoma regressed spontaneously and that a new melanoma lesion (the thumbnail melanoma) was detected and was one of multiple primary melanomas. The incidence of multiple primary melanomas is 1~8% and the incidence of ALM in Asians is higher than in other ethnic groups; in Korea, the most common type of melanoma is ALM8. However, ALM is associated with a lower incidence of multiple primary melanomas than sporadic nonacral melanoma9,10. To the best of our knowledge, there are no available studies demonstrating an increased incidence of developing a second melanoma on an acral site in Asian patients.
Although it was impossible to confirm that the melanoma of the left thumb was the primary site, we consider this case interesting because it helps us to understand the pathogenesis of MUP and reminds physicians to conduct careful periodical work-ups of melanoma patients; it also highlights the importance of continued long-term follow-up, especially in patients with MUP.
Figures and Tables
Fig. 1
There is a black patch on the proximal nail fold and melanonychia of the left first fingernail.
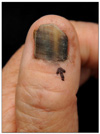
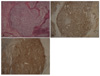
Fig. 2
(A) The biopsy specimen from the left first finger shows acanthosis, rete ridge elongation, and atypical melanocytes along the dermo-epidermal junction (H&E, ×100). The atypical melanocytes react positively to (B) HMB-45 (HMB-45, ×100) and (C) S-100 (S-100, ×100).

References
1. Anbari KK, Schuchter LM, Bucky LP, Mick R, Synnestvedt M, Guerry D 4th, et al. Melanoma of unknown primary site: presentation, treatment, and prognosis--a single institution study. University of Pennsylvania Pigmented Lesion Study Group. Cancer. 1997. 79:1816–1821.
2. Ceballos PI, Barnhill RL. Spontaneous regression of cutaneous tumors. Adv Dermatol. 1993. 8:229–261.
3. Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992. 20:315–322.

4. Mahrle C, Bolling R, Gartmann H. Verrucous malignant melanoma (spontaneous regression and simultaneous development of a secondary tumor). Z Hautkr. 1977. 52:897–905.
5. Lipsker D, Engel F, Cribier B, Velten M, Hedelin G. Trends in melanoma epidemiology suggest three different types of melanoma. Br J Dermatol. 2007. 157:338–343.

6. Kwon IH, Lee JH, Cho KH. Acral lentiginous melanoma in situ: a study of nine cases. Am J Dermatopathol. 2004. 26:285–289.

7. Cohen-Solal KA, Crespo-Carbone SM, Namkoong J, Mackason KR, Roberts KG, Reuhl KR, et al. Progressive appearance of pigmentation in amelanotic melanoma lesions. Pigment Cell Res. 2002. 15:282–289.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download