Abstract
Granulocytic sarcoma is an extramedullary tumor composed of granulocytic precursor cells. It usually presents as a nodular mass in the course of acute myelogenous leukemia. Rarely, the tumor develops in non-hematological conditions or in a patient with complete remission from the acute myelogenous leukemia. In such cases, aleukemic granulocytic sarcoma can be a preceding sign of systemic leukemia or a first sign of hematologic relapse of leukemia. We present an unusual case of multiple granulocytic sarcomas developed in a patient with longstanding complete remission of acute myelogenous leukemia, who has not had bone marrow and peripheral blood involvement for a long time.
Granulocytic sarcomas usually occur during the course of acute myelogenous leukemia (AML) and antecede blastic cirisis in myeloproliferative disease or myelodysplastic syndromes. However, the tumor can also develop in patients who have either no prior history of hematological disease or have shown complete remission from acute myelogenous leukemia.
Herein, we describe a patient who has shown an unusual clinical course in that she was in complete remission from AML for a long time and that there were no signs of peripheral blood and bone marrow relapse for a long time following the development of granulocytic sarcoma.
A 72-year-old Korean woman presented with a 1-month history of rapidly growing multiple cutaneous nodules on the whole body. Physical examination revealed widespread well-demarcated variable-sized greenish firm cutaneous nodules over the face, trunk and extremities (Fig. 1).
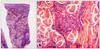
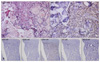
She had a history of acute myeloblastic leukemia without maturation (AML M1), and achieved complete remission after the 3rd consolidation chemotherapy, ten years ago. The patient remained asymptomatic for nine years and then visited the hospital presenting with an asymptomatic solitary firm mass on the left upper eyelid in September 2009. An excisional biopsy of the lesion was performed and histopathologic findings were consistent with a granulocytic sarcoma. Bone marrow biopsy showed normocellular bone marrow without leukemic cell involvement and peripheral blood also revealed normal findings. In April 2010, she complained of nasal stuffiness, bloody nasal discharge and hoarseness. Neck computed tomography and magnetic resonance imaging findings showed a mass in the nasopharynx. Histopathologic findings of this lesion are also consistent with granulocytic sarcoma. At that time, there was no systemic involvement of leukemia. Although she was referred to the Department of Hematology, she refused systemic treatment. In September 2010, she visited our clinic due to the multiple skin lesions. On laboratory examination, the white blood cell count was 6·2×109 L-1 with 69% segmented neutrophils, 24% lymphocytes, 7% monocytes, 0% eosinophils and 0% basophils, without evidence of abnormal cells. The red blood cell count, platelet count and hemoglobin level were all within the normal range. Bone marrow biopsy showed a normocellular bone marrow without leukemic cell involvement. Histopathological examination of a skin biopsy specimen from the trunk revealed a dense cellular infiltrate among collagen bundles and in the perifollicular areas within the entire dermis (Fig. 2A). The infiltrate consisted of homogenous atypical cells with round to oval hyperchromatic nuclei (Fig. 2B). Tumor cells are positive for Leder stain and immunoperoxidase (Fig. 3A), but negative for CD3, CD20, CD34, TdT and Pax-5 (Fig. 3B).
From pathological and immunohistological findings, we confirmed the diagnosis of granulocytic sarcoma for the skin lesions. The patient was transferred to hematology again for proper management, but she refused the treatment for personal reasons.
Granulocytic sarcoma is an extramedullary tumor composed of granulocytic precursor cells, such as myeloblast or immature myeloid cells. It is also known as myeloid sarcoma, extramedullary tumor and chloroma. Granulocytic sarcoma clinically manifests as a solid nodular mass, which commonly affects the bone, periosteum, lymph node and skin. Granulocytic sarcomas usually occur during the course of AML and antecede blastic cirisis in myeloproliferative disease or myelodysplastic syndromes1-5. However, the tumor can also develop in patients who have either no prior history of hematological disease or have shown complete remission from acute myelogenous leukemia6-11. Aleukemic granulocytic sarcoma is very rare. It usually precedes systemic relapse in most cases.
The pathogenesis of aleukemic granulocytic sarcoma is yet to be elucidated. Takahashi et al.12 suggested that clonal evolution might be related to conversion of the character of leukemic blasts. Up-regulating expression of some adhesion molecules in the AML-M3 patient treated with all-trans retinoic acid was also hypothesized7. However, our patient had a history of AML-M1, and cytogenetic analysis of the granulocytic sarcoma revealed no chromosomal abnormality with 46, XX. These facts do not fit with the above conditions. In terms of a molecular basis, the homing of T cells into the skin via interaction with integrins and endothelial-bound chemokines is suggested as a mechanism of extramedullary invasion of leukemic cells13.
In the diagnosis of granulocytic sarcoma, histopathologic examination is necessary. Immunohistochemical studies of the lesions and history of underlying systemic leukemia are helpful in differential diagnosis with lymphoma, mycosis fungoides and other neoplasms. In a retrospective review, Cronin et al.14 documented that myeloperoxidase expression strongly supports the diagnosis of myeloid leukemia cutis.
The treatment of aleukemic granulocytic sarcoma has not been established. Chemotherapy or radiotherapy is usually recommended. Several studies have suggested that aggressive chemotherapy at the time of diagnosis of aleukaemic granulocytic sarcoma may improve the overall survival rate and help prevent subsequent progression to AML15,16.
The prognosis of aleukemic granulocytic sarcoma is poor. In such cases, relapse of leukemia occurs within a mean of 10 months following granulocytic sarcoma1,9. Rarely, overt leukemia can occur after a long period since the onset of granulocytic sarcoma17.
Our patient has shown an unusual clinical course in that she was in complete remission from AML for a long time and that there were no signs of systemic involvement for a long time following the development of granulocytic sarcoma. She remained asymptomatic for a long time after achieving complete remission of AML M1. Nine years later, she presented with multiple granulocytic sarcoma. Although cutaneous multiple lesions show a tendency to progress rapidly, no systemic leukemic involvement has been observed for seventeen months of the follow-up period.
From our case, we see that multiple granulocytic sarcomas can develop even in patients who have shown complete remission of AML for an extended period of time. Therefore, we think that careful periodic follow-up including hematologic evaluation is needed for such patients as aleukemic granulocytic sarcoma can be a first sign of hematologic relapse of leukemia.
Figures and Tables
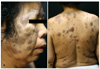 | Fig. 1(A, B) Widespread well-demarcated variable-sized greenish firm cutaneous nodules over the face, trunk and extremities. |
References
1. Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981. 48:1426–1437.

3. Duguid JK, Mackie MJ, McVerry BA. Skin infiltration associated with chronic myelomonocytic leukaemia. Br J Haematol. 1983. 53:257–264.

4. Murakami Y, Nagae S, Matsuishi E, Irie K, Furue M. A case of CD56+ cutaneous aleukaemic granulocytic sarcoma with myelodysplastic syndrome. Br J Dermatol. 2000. 143:587–590.

5. de Arruda Câmara VM, Morais JC, Portugal R, da Silva Carneiro SC, Ramos-e-Silva M. Cutaneous granulocytic sarcoma in myelodysplastic syndrome. J Cutan Pathol. 2008. 35:876–879.

6. Long JC, Mihm MC. Multiple granulocytic tumors of the skin: report of six cases of myelogenous leukemia with initial manifestations in the skin. Cancer. 1977. 39:2004–2016.

7. Firat F, Ozen G, Yildiz G, Sağlam A, Onder S, Tan E, et al. Late relapse of acute myeloblastic leukemia as myeloid sarcoma causing radiculopathy. Leuk Res. 2010. 34:e348–e350.
8. Byrd JC, Weiss RB. Recurrent granulocytic sarcoma. An unusual variation of acute myelogenous leukemia associated with 8;21 chromosomal translocation and blast expression of the neural cell adhesion molecule. Cancer. 1994. 73:2107–2112.

9. Harris DW, Ostlere LS, Rustin MH. Cutaneous granulocytic sarcoma (chloroma) presenting as the first sign of relapse following autologous bone marrow transplantation for acute myeloid leukaemia. Br J Dermatol. 1992. 127:182–184.

10. Orlandi E, Morra E, Lazzarino M, Castagnola C, Paulli M, Rosso R, et al. Multiple granulocytic sarcoma during complete hematologic remission of acute nonlymphoid leukemia. Acta Haematol. 1989. 81:41–43.

11. Mason TE, Demaree RS Jr, Margolis CI. Granulocytic sarcoma (chloroma), two years preceding myelogenous leukemia. Cancer. 1973. 31:423–432.

12. Takahashi T, Tsukuda H, Kimura H, Yoshimoto M, Tsujisaki M. Extramedullary relapse of AML with t(9;11)(p22;q23) associated with clonal evolution from trisomy 8 into tetrasomy 8. Intern Med. 2010. 49:447–451.

13. Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol. 2008. 129:130–142.

14. Cronin DM, George TI, Sundram UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009. 132:101–110.

15. Imrie KR, Kovacs MJ, Selby D, Lipton J, Patterson BJ, Pantalony D, et al. Isolated chloroma: the effect of early antileukemic therapy. Ann Intern Med. 1995. 123:351–353.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download