Abstract
Aeromonas hydrophila is a facultatively anaerobic, asporogenous gram-negative rod that has often been regarded as an opportunistic pathogen in hosts with impairment of a local or general defense mechanism. A 68-year-old alcoholic woman presented with shock and gangrene on the right arm. At first, her clinical presentations were severe painful erythematous swelling that worsened within a few hours with development of gangrene, edema, and blisters. Bullous fluid and blood cultures yielded A. hydrophila. Histopathological findings of sections obtained from the vesicle revealed subepidermal vesicles; necrosis of the epidermis, papillary dermis, and subcutaneous fat; and massive hemorrhage in the subcutis. Despite all efforts to save the patient, she died 8 hours after admission. Clinical features of A. hydrophila sepsis resemble those of Vibrio vulnificus sepsis. Therefore, in addition to the case report, we compared the cultural, biochemical, and morphological differences between A. hydrophila and V. vulnificus for facilitation of early and accurate identification of the causative agent.
Aeromonas species are widely distributed in stagnant and flowing fresh waters, at the interface of sea water and fresh water, and in sewage. They have also been isolated from soil and foodstuffs1. Aeromonas hydrophila (A. hydrophila) has often been recognized as an opportunistic pathogen in hosts with impairment of a local or general defense mechanism. According to the literature, the following 4 categories of infection have been described: cellulitis, acute diarrheal disease, sepsis, and other infections2,3.
Here, we report on a clinical syndrome resulting from infection with A. hydrophila, which was indistinguishable from the one usually associated with infection due to Vibrio vulnificus (V. vulnificus). V. vulnificus sepsis is a primary sepsis syndrome that can develop in individuals with preexisting chronic hepatic disease after consumption of raw seafood. It usually occurs during the summer, and, in Korea, it is characterized by high mortality (62.4%)4. We conducted a brief comparison of cultural, biochemical, and morphological characteristics between A. hydrophila and V. vulnificus sepsis for early and accurate identification of the causative pathogen.
In September, a 68-year-old woman visited our emergency department with a 1-day history of tense necrotic vesicobullous lesions over the cyanotic skin of the right arm. On the morning of the admission day, the patient experienced sudden severe pain in the right upper extremity. Over the next several hours, she developed painful erythematous swelling and vesicles, which showed gradual progression to hemorrhagic bullae and ecchymoses. Her medical history was not remarkable; however, she had not undergone regular health checkups. In addition, she had a 20-year history of alcohol abuse (750 ml of Korean rice wine per day). However, whether or not she had eaten raw fish was not certain.
Physical examination revealed slightly icteric sclera and edema, cyanosis, and dusky purplish discoloration with necrotic tense vesicobullous lesions on the right arm (Fig. 1). She became comatose while being transferred from a private clinic to our hospital. Her pupils were completely dilated, and her blood pressure could not be checked because of low pressure. She was in a state of cardiopulmonary arrest. After approximately 30 minutes of active cardiopulmonary resuscitation and medical therapy for treatment of shock, her vital signs became stabilized. After 40 minutes, we started intravenous injection of ampicillin and cephalosporin. However, she died at 1 hour after administration of antibiotic therapy.
Her white blood cell count was 4,000/µl with 50% neutrophils, hemoglobin level was 12.8 g/dl, and platelet count was 350,000/µl. Levels of serum alkaline phosphatase, aspartate aminotransferase, and alanine aminotransferase were 326 IU/L, 528 IU/L, and 208 IU/L, respectively. Total serum protein level was 2.9 g/dl, with an albumin level of 1.4 g/dl. Bacterial cultures obtained from blood and bullous fluid were positive for A. hydrophila.
A skin biopsy specimen obtained from a vesicle revealed a subepidermal vesicle; necrosis of the epidermis, papillary dermis, and subcutaneous fat; and massive hemorrhage in the subcutis (Fig. 2).
Using 16 strains of V. vulnificus and 1 strain of A. hydrophila isolated from blood and/or bullous fluid of patients with primary sepsis, we studied the culture characteristics of the strains on 8 types of media that are commonly used in clinical laboratories. Media used were blood and thiosulfate-citrate-bile salts-sucrose (TCBS) for Vibrionaceae, bile esculin azide (BEA) for Group D Streptococci, and selective isolation media for Enterobacteriaceae, such as MacConkey, eosin methylene blue (EMB), Hektoen enteric (HE), Salmonella-Shigella (SS), and Endo agars. All strains were cultured at 37℃ for 24 hours at. Culture results are summarized in Table 1.
In all, 33 tests were performed for obtaining differential data. We tested 1 A. hydrophila strain and 16 V. vulnificus strains. Results are summarized in Table 2.
A. hydrophila were gram-negative straight bacilli, whereas V. vulnificus were gram-negative curved bacilli (Fig. 3).
In general, the size of A. hydrophila was smaller than that of V. vulnificus. A. hydrophila had a rough cell wall and a relatively wide periplasmic space, compared with V. vulnificus (Fig. 4).
A. hydrophila was sensitive to chloramphenicol, cefamandole nafate, netilmicin sulfate, gentamicin, amikacin sulfate, and ceftriaxone sodium and was resistant to ampicillin, carbenicillin, and cephalothin.
V. vulnificus (all 16 strains) were sensitive to ampicillin, tobramycin, erythromycin, piperacillin, chloramphenicol, and cephalosporins (third generation).
Primary sources of clinical isolates of Aeromonas species include stool, wound, abscess, cellulitis, and blood5. In a normal human host, infection with A. hydrophila may produce mild diarrhea and, occasionally, cellulitis. However, severe Aeromonas infection occurs when host defenses are compromised. The majority of patients with A. hydrophila sepsis have a history of leukemia, other malignant diseases, or hepatobiliary diseases6.
Among the many bacteria responsible for causing sepsis with skin lesions, V. vulnificus and A. hydrophila have the capacity to cause illness and skin lesions in compromised hosts; the infections caused by these 2 strains are clinically indistinguishable from one another. The clinical features of our patient were similar to those observed in patients with V. vulnificus sepsis.
In Korea, most patients with V. vulnificus infection have preexisting hepatic disease or history of alcohol abuse, and V. vulnificus sepsis almost always occurs during the summer (between July and September)4. Therefore, because the patient was severely alcoholic and had visited our hospital in September, we diagnosed this patient as having sepsis due to V. vulnificus.
Of the many exotoxic factors produced by A. hydrophila, endotoxin and β-hemolysin have been known to contribute to pathologic and cutaneous findings7,8. Sepsis caused by A. hydrophila and by V. vulnificus, despite the many morphological, cultural, and biochemical dissimilarities between the 2 bacterial strains, show clinical similarities with regard to cutaneous lesions, such as hemorrhagic necrosis, edema, blisters, and a fulminant fatal course in patients with a history of alcohol abuse, hepatic disease, and malignant disease.
Gram stain, culture, and biochemical tests are used for identification of A. hydrophila. Use of these methods is the best way to confirm diagnosis; however, they are fastidious and time consuming. A polymerase chain reaction (PCR) method that is convenient, rapid, and specific for identification of pathogens has recently been developed9. However, due to lack of facilities, this method was not used for our case.
Results of our antibiotic sensitivity test on A. hydrophila were consistent with those reported by other researchers6,10. Gentamicin, chloramphenicol, and tetracycline would appear to be the treatment of choice. Unlike V. vulnificus, A. hydrophila is known to be resistant to ampicillin and erythromycin. Since the antibiotic susceptibility patterns of the 2 bacterial strains differ, we recommend the use of chloramphenicol or third generation cephalosporins as the first drug of choice for patients in whom infection caused by one of these two bacteria is suspected.
Some papers have reported on A. hydrophila sepsis in the Korean literature11-13. Kang et al.11 evaluated the clinical significance of Aeromonas bacteremia in 182 patients. Most cases (75.8%) developed during summer and autumn, and half of the patients (48.9%) suffered from liver cirrohosis. Mortality directly related to Aeromonas sepsis was 24.1% and was associated with old age, skin and soft tissue infection, septic shock, and altered consciousness. Ha et al.12 reported on a patient with alcoholic cirrohosis, and Park et al.13 reported one who had undergone prolonged hemodialysis. In our case, a-68-year-old patient with a heavy alcoholic history showed skin lesion, sepsis, and comatous mental status, and finally died. Impairment of defense mechanisms, such as lack of significant opsonizing activity against the autologous infecting strain may serve to explain some of the pathogenesis of Aeromonas infection on immunocompromised patients14.
In conclusion, we have presented a case of an alcoholic woman with sepsis and gangrene caused by A. hydrophila, often confused with V. vulnificus. Despite clinical similarities between the two strains, distinction of the type of strain responsible for the infection is important. Due to different antibiotic susceptibilities, early and accurate identification of the causative pathogen is essential to achievement of better outcomes.
Figures and Tables
Fig. 1
Edema, cyanosis, and dusky purplish discoloration with necrotic tense blisters on the right upper extremity.
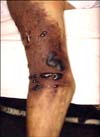
Fig. 2
Biopsy specimen obtained from the vesicle revealed subepidermal vesicle, necrosis of the epidermis, papillary dermis and subcutaneous fat, and massive hemorrhages in the subcutis (H&E, ×40).
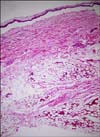
Fig. 3
Gram staining of culture smears of Aeromonas hydrophila (A) and Vibrio Vulnificus (B) showing gram-negative straight rods and gram-negative curved rods, respectively (Gram stain, ×1000).

Fig. 4
Electron micrograph of Aeromonas hydrophila (A) stained with ruthenium red showing straight rods and division septum, compared with the morphology of Vibrio vulnificus (B) showing curved bacilli (×13000).

References
1. Abbott SL. Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Aeromonas. Manual of clinical microbiology. 2003. 8th ed. Washington DC: ASM Press;701–705.
2. Davis WA 2nd, Kane JG, Garagusi VF. Human Aeromonas infections: a review of the literature and a case report of endocarditis. Medicine (Baltimore). 1978. 57:267–277.
3. Janda JM, Abbott SL. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010. 23:35–73.

4. Park SD, Lee JY, Kim HD, Yoon NH. Clinical study of vibrio vulnificus sepsis. Korean J Dermatol. 2006. 44:696–707.
5. McCracken AW, Barkley R. Isolation of Aeromonas species from clinical sources. J Clin Pathol. 1972. 25:970–975.

6. Trust TJ, Chipman DC. Clinical involvement of Aeromonas hydrophila. Can Med Assoc J. 1979. 120:942–946.
7. Thelestam M, Ljungh A. Membrane-damaging and cytotoxic effects on human fibroblasts of alpha- and beta-hemolysins from Aeromonas hydrophila. Infect Immun. 1981. 34:949–956.

8. Brenden RA, Huizinga HW. Pathophysiology of experimental Aeromonas hydrophila infection in mice. J Med Microbiol. 1986. 21:311–317.

9. Trakhna F, Harf-Monteil C, Abdelnour A, Maaroufi A, Gadonna-Widehem P. Rapid Aeromonas hydrophila identification by TaqMan PCR assay: comparison with a phenotypic method. Lett Appl Microbiol. 2009. 49:186–190.

10. Von Graevenitz A, Mensch AH. The genus Aeromonas in human bacteriology report of 30 cases and review of the literature. N Engl J Med. 1968. 278:245–249.
11. Kang JM, Kim BN, Choi SH, Kim NJ, Woo JH, Ryu JS, et al. Clinical features and prognostic factors of Aeromonas bacteremia. Infect Chemother. 2005. 37:161–166.
12. Ha BS, Won YH, Chun IK, Kim YP. Haemorrhagic gangrene of skin caused by Aeromonas hydrophila. Ann Dermatol. 1989. 1:98–101.

13. Park HJ, Kim HY, Uh Y, Kwon OG, Oh JR. A case of Aeromonas hydrophila necrotizing fasciitis in patient on hemodialysis. Infect Chemother. 2007. 39:218–221.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download