Abstract
Connective tissue nevus is not a true tumor, but rather a hamartoma involving various components of connective tissue. It presents as a slow-growing, painless, flesh-colored, or pink nodule or plaque that is evident from childhood. While any region of the body may be affected, there is a predilection for the trunk and extremities. A 20-month-old girl presented with three ipsilateral confluent popular plaques with zosteriform distribution that had formed over the previous 17 months on the left chest and abdomen. The patient remained asymptomatic. Unlike all previously reported cases demonstrating a single lesion, we report a connective tissue nevi in a child who presented with multiple unilateral zosteriform lesions, an unusual pattern of distribution without evidence of tuberous sclerosis complex.
Connective tissue nevus (CTN) is a hamartoma of various components of dermal connective tissue elements, including collagen, elastin, or glycosaminoglycan, showing either an increase, normal range, or decrease in elastic fiber, with an increase in collagen fiber1. Clinically, it presents with a "peau de chagrin" or cobblestone-like plaque2. Zosteriform CTN was first described by Steiner in 1944, presenting with grouped papules in a zosteriform band without extracutaneous manifestation3. Several reported cases of zosteriform CTN have been found in the worldwide literature1,3-6.
We present an interesting case of a child with three unilateral zosteriform CTN on the trunk.
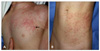
A 20-month-old girl presented with a progressively increasing number of multiple asymptomatic papules on the left side of the chest (C4, T1-3) and abdomen (T11, 12) that had appeared over the previous 17 months. Past medical history was unremarkable, with no sign or record of congenital defect. The patient had no family history of similar cutaneous lesions or abnormal disorders involving the central nervous system. Examination of the skin found three ipsilateral segmental plaques with hundreds of confluent papules distributed in a zosteriform pattern on the left chest and abdomen (Fig. 1). Normal segments of skin intervened between the two zosteriform plaques.
Plaques and papules had an erythematous to skin-colored tint and soft consistency. Among the numerous papules with a diameter of 2 to 4 mm, several larger (6 to 8 mm), discrete, dome-shaped papules were found. A complete inspection revealed none of the Café-au-lait macules, axillary freckling, hypopigmented macules, periungual fibromas, gingival fibromas, or dental pitting. Neurological and ophthalmological examinations revealed no abnormal findings, and no laboratory abnormalities were observed. Other tests included chest X-ray, electrocardiogram, cranial X-ray, and magnetic resonance imaging. Results were within normal limits. Genetic study was not available.
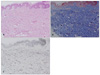
A skin punch biopsy that included one of the domeshaped papules was performed. Histopathologic examination showed normal epidermis and thickened collagen fibers in the dermis, with poor inflammatory elements (Fig. 2A). Masson-Trichrome staining for collagen revealed abundant proliferation of collagen fibers (Fig. 2B). Elastic staining showed decreased elastic tissues in the dermis (Fig. 2C). The histopathologic finding of a polypoid portion revealed prominent dermal dilated vessels with mild fibrosis in the upper and middle dermis, dense collagen bundles. Based on these findings, a diagnosis of CTN was established.
CTN, first reported by Lewandowsky in 19217, may occur as either an isolated finding with no familial history, or as an inherited autosomal dominant trait without disorders in other organs8, and it could also be accompanied by a genetic disorder, such as familial cutaneous collagenoma, tuberous sclerosis complex (TSC), Buschke-Ollendorff syndrome, or osteopoikilosis9,10.
CTN is characterized by irregular increase of collagen fibers in the dermis and absence of an increase in fibroblasts. The generally accepted theory concerning pathogenesis of CTN is a disorder in the process of collagen, elastic fiber, and mucopolysaccharide synthesis11. Theologically, excessive accumulation of collagen fibers could result either from locally increased production or decreased degradation of collagen in lesions12. However, the theory is not applicable to all cases diagnosed as CTN.
Zosteriform CTN, which displays a segmental distribution pattern, is rare, especially in the variety with a predominence of collagen5. It was first reported in 1944 by Steiner3, who described the case of a 5-year-old girl with unilateral distribution located on the right side of the lower chest and back. The patient had no other associated findings, and no familial history of abnormal cutaneous eruptions or abnormal neurological disorders. Five cases of zosteriform CTN have been reported in the English literature1,3-6 (Table 1). Similar to our case, three of them were zosteriform CTN involving more than two segments of skin3,5,6. Asano et al.13 also reported on a case of multiple CTN distributed as multiple linear streaks, however, it was along Blaschko lines, and unrelated to dermatomal lines.
Very little is known about the etiology of zosteriform CTN. Whether zosteriform CTN is a segmental form of TSC or a distinct category of CTN is debatable. Yeh et al.5, however, emphasized that ZCTN should be considered as a separate entity because of its unusual clinical presentation, the lack of a genetic pattern of inheritance, and the absence of associated abnormalities in other organs. Unfortunately, we were unable to perform genetic tests, and could not clarify this suspicion.
Clinically, zosteriform CTN has similar morphology and distribution to nevus lipomatosus superficialis (NLS) or segmental neurofibromatosis. In typical NLS, skin-colored soft papules or nodules are linearly distributed on the hip or buttock. However, the histopathologic findings of NLS make it easy to differentiate from zosteriform CTN14. Because segmental neurofibromatosis could also involve multiple segments of skin15, differential diagnosis with segmental neurofibromatosis was very important in our case. Two disorders, however, could be easily differentiated by different histopathologic findings.
In our case, dome-shaped papules were simultaneously found on the plaque of CTN. Histologically, it showed dermal dilated vessels with mild fibrosis in the dermis and dense collagen bundles. However, we suppose that these findings were coincidental findings.
There is no specific management strategy. However, careful periodic and close follow-up is required to establish a definite diagnosis, and to monitor disease progression, or to detect any systemic complications that may occur in a minority of patients.
We report on an interesting case of multiple unilateral zosteriform CTN with no other evidence of genetic disease.
Figures and Tables
Fig. 1
(A) Two (C4, T1~3) ipsilateral segmental erythematous to flesh-colored plaques with numerous confluent papules and several discrete dome-shaped papules (arrow) on the left anterior chest. (B) The third (T11~12) segmental lesion on the abdomen.
References
1. Amjadi M, Khorrami-Arani N, Mashman G, Allen PW. Zosteriform connective tissue nevus: a case report. Am J Dermatopathol. 2007. 29:303–305.

2. Atherton DJ. Champion RH, Burton JL, Burns DA, Breathnach SM, editors. Naevi and other developmental defects. Rook/Wilkinson/Ebling textbook of dermatology. 1998. 6th ed. Oxford: Blackwell Science;519–616.

4. Kozminsky ME, Bronson DM, Barsky S. Zosteriform connectivetissue nevus. Cutis. 1985. 36:77–78.
5. Yeh SW, Magalhaes AM, Vasconcellos MR, Michalany NS, Tomimori Yamashita J. Zosteriform connective tissue nevus: a case report. Int J Dermatol. 2003. 42:720–722.

6. Brazzelli V, Muzio F, Barbagallo T, Fornara L, Donadini F, Guerci B, et al. Zosteriform connective tissue nevus in a pediatric patient. Pediatr Dermatol. 2007. 24:557–558.

7. Lewandowsky F. Uber einem eigentumlichen Neuus der Brustgegend. Arch Dermatol Syphilol. 1921. 131:90–94.
8. Uitto J, Santa-Cruz DJ, Eisen AZ. Familial cutaneous collagenoma: genetic studies on a family. Br J Dermatol. 1979. 101:185–195.

9. Raque CJ, Wood MG. Connective-tissue nevus. Dermatofibrosis lenticularis disseminata with osteopoikilosis. Arch Dermatol. 1970. 102:390–396.

10. Schorr WF, Optiz JM, Reyes CN. The connective tissue nevus-osteopoikilosis syndrome. Arch Dermatol. 1972. 106:208–214.

11. Herbst VP, Kauh YC, Luscombe HA. Connective tissue nevus masquerading as a localized linear epidermal nevus. J Am Acad Dermatol. 1987. 16:264–266.

12. Uitto J, Bauer EA, Santa Cruz DJ, Holtmann B, Eisen AZ. Decreased collagenase production by regional fibroblasts cultured from skin of a patient with connective tissue nevi of the collagen type. J Invest Dermatol. 1982. 78:136–140.

14. Mehregan AH, Tavafoghi V, Ghandchi A. Nevus lipomatosus cutaneous superficialis (Hoffmann-Zurhelle). J Cutan Pathol. 1975. 2:307–313.

15. Kumar S, Kumar RP. Multi-segmental neurofibromatosis. Indian J Dermatol Venereol Leprol. 2004. 70:361–363.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download