Abstract
Myeloid sarcoma is a tumor which consists of myeloblasts or immature myeloid cells. This tumor presents in the lymphoid organs, bone, skin, soft tissue, various mucosae and organs, and the central nervous system. Granulocytic sarcoma, an extramedullary acute myeloid leukemia, is also referred to as chloroma (GS) because of its greenish surface color. Granulocytic sarcoma is rare and difficult to diagnose. We can easily misdiagnose this tumor as lymphoma or sarcoma, especially when there is no evidence of hematologic disorders. Immunohistochemical studies are helpful in determining the correct diagnosis. Antibodies to myeloperoxidase, lysozyme, and chloroacetate esterase are used for the diagnosis of granulocytic sarcoma. In addition, detection of cell surface markers such as CD 33, CD 34, CD 68, CD 99, and HLA-DR may be useful. We describe a case of GS that presented with bluish nodules on the right cheek of a 54-year-old woman with immunohistochemical findings for correct diagnosis.
Myeloid sarcoma refers to a tumor formed by myeloblasts or immature myeloid cells that affects an extramedullary site or soft tissue, such as the skin. The mass, also referred to as an extramedullary myeloid tumor, granulocytic sarcoma, or chloroma, may precede aleukemic leukemia, or present simultaneously with a systemic myeloproliferative disorder or represent a relapse of a disease previously in remission1. The term 'chloroma' has been used to describe this disease due to the green hue emitted by the myeloperoxidase (MPO) produced by these tumor cells2. However, granulocytic sarcoma (GS) is the preferred term nowadays because not all of these tumors are green3. Herein, we report an unusual case of GS of the face in a patient without leukemia.
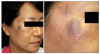
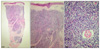
A 54-year-old female patient presented in July 2009 with several facial lesions located on the right cheek for the past 4 years. The lesions were 0.5~1.0 cm-sized light brownish to bluish nodules and a 2.0 cm-sized bluish plaque (Fig. 1). She had suffered from nasal congestion for about 1 year. There were a few polyps in the nasal cavity which were biopsied at another clinic. The biopsy tissues were diagnosed as myeloid sarcoma in the nasal cavity and were completely removed by excision several weeks earlier at our hospital. The paranasal sinus computed tomography results before excision showed granulation tissue in the nasal cavities. Following histopathologic examination, the excision tissues from the nasal cavities were revealed to be myeloid sarcoma. Hematologic study showed a leukocyte count of 4,870/µl with normal differential count. Bone marrow aspiration results showed no evidence of increased blast count. Histopathologic examination of the skin lesions revealed atypical lymphoproliferation (Fig. 2). The results of immunohistochemistries for CD 3, CD 4, CD 8, CD 20, CD 23, and CD 5a, which are B- and T-cell markers, were negative. The results showed that the tumor was not lymphoma. We also attempted further immunohistochemical examination with antibodies to MPO, CD 68, and CD 99 (Fig. 3), and the results were all positive. From these findings we diagnosed these lesions as granulocytic sarcoma. The patient was transferred to the hematologic department and treated with idarubicin and cytarabin, which are used for acute myelogenous leukemia (AML) treatment. One year after induction and consolidation chemotherapy, hematologic analysis revealed a leukocyte count of 2,490/µl with no evidence of systemic leukemia and relapsed cutaneous tumor.
GS is an extramedullary leukemia tumor, that includes leukemia cutis and meningeal leukemia. GS is characterized by localized infiltration of immature granulocytes in an extramedullary site or soft tissue. GS was first described in the early 1800s as a tumor on the retro-orbital site with a green-colored appearance, which caused proptosis4. The term "chloroma" is derived from the Greek word chloros (green) and was used by King2 in 1853. In 1967, Rappaport3 referred this tumor as GS because not all of the tumors were green.
In many cases, GS occurs in childhood with one rapid-growing mass, but also presents as multiple lesions. It is generally seen in the ribs, sternum, pelvis, orbital bone, soft tissues, lymph nodes, skin, and gums. It also involves the spine, small intestine, orbit, cervix, and nasal sinuses. GS occurs at any age, but some reports suggest a wide age range with a mean onset during middle age5. There is no sexual predilection for GS.
Clinically, GS can occur in the following three scenarios: 1) AML or other bone marrow and blood involvement, 2) isolated recurrence of AML or as a sign of blast transformation in patients with chronic myelogenous leukemia (CML), and 3) before the presence of systemic leukemia, as a harbinger for AML6. GS is found in only 3% to 8% of leukemia patients and is associated with AML and chronic myeloproliferative disorder (CMPD). It can also occur as a precursor to AML rarely without any evidence of a hematological disorder7. The primary GS is a rare condition that occurs in the absence of any past history of leukemia and is commonly misdiagnosed as lymphoma or sarcoma. One report showed that misdiagnosis occurred in 47% (72 out of 154 cases of primary extramedullary leukemia) of cases, and another study reported an incident rate of 56% (34 cases in 61 biopies of 50 patients) of misdiagnosis8. Thus, immunohistochemical studies must be evaluated for correct diagnosis.
Histologically, GS undergoes various morphological changes in the cells. It is composed of immature cells of the neutrophil granulocytic series. The infiltration of immature, poorly differentiated cells with round to oval nuclei is the predominant finding. Crystalline, rod-like, intracytoplasmic acidophilic bodies called Auer rods are sometimes seen9. With these histological findings, it is hard to distinguish GS from other lymphomas including histiocytic lymphoma, lymphoblastic lymphoma, Ewing's sarcoma, lymphocytic leukemia, undifferentiated carcinomas, and primitive neuroectodermal tumors10. B-and T-cell markers (CD 45, CD 20, UCHL-1, CD 3, and CD 30) would be helpful to rule out a diagnosis of lymphoma. Definitive diagnosis requires histochemical stains to demonstrate the presence of MPO. Anti-lysozyme and chloroacetate esterase can also be used to diagnose GS. Sudan black B, peroxidase or diaminobenzidine reactions are used to identify MPO. MPO is localized in the primary granules of the myeloid cells and is synthesized early in differentiation, thus serving as an important marker for myeloid lineage11. Other monoclonal antibodies to the membrane surface or cytoplasmic antigens such as CD 33, CD 34, CD 68, CD 99, and HLA-DR can also be useful12. In our case, positive results were obtained for antibodies to myeloid cells such as anti-MPO, anti-CD 68, and anti-CD 99. MPO is a valuable marker and a positive outcome means that this tumor is a myeloblastic variant. CD 68 positive shows that the tumor cells are of monocytic and granulocytic lineages. CD 99 is expressed by a significant proportion of myeloid sarcomas. Additionally, a positive TdT stain indicates immature or nondifferentiated tumor cells. A special stain for Leder Reaction can result in a focally positive outcome. We were easily able to diagnose GS with these immunohistochemical results.
There are multiple predisposing factors for the development of primary and secondary GS. Cytogenetic abnormalities including t(8;21), inv(16), FAB subtypes M4 and M5, high white blood cell count, the presence of NCAM and/or T-cell markers (CD 2, CD 4, or CD 7), and poor nutrition and low socioeconomic status have been suggested to associate with GS development8.
The prognosis of GS is not very good. Its course is rapid and it has a high mortality rate, especially when associated with AML13. Patients without evidence of leukemia have a better outcome. Cases initially diagnosed as GS without evidence of leukemia and treated with systemic chemotherapy are likely to have a lower probability of developing acute myeloid leukemia and were shown to be associated with longer survival rates14. However, patients who received no chemotherapy had a higher chance of developing leukemia after diagnosis of GS without hematologic abnormality at the initial diagnosis15. Furthermore, early systemic therapy was shown to be helpful to prevent AML development and resulted in longer overall survival14. Therefore, GS should be treated early in the course of the disease with systemic treatment. Irradiation therapy is also used for primary GS. Although radiotherapy is effective for high radiosensitivity, it does not improve disease free survival and prognosis. It is suggested that GS is a systemic disease and resection or irradiation treatment should be combined with intensive chemotherapy16. In the Korean literature, three cases of cutaneous GS have been reported17,18. In two such cases, skin lesions developed in patients with AML, and in another case, cutaneous GS was found before the presence of systemic leukemia17,18.
We clinically experienced a case of GS arising on the face, and there was no association with leukemia. Immunohistochemical studies have been helpful for making the correct diagnosis. Primary GS may be a harbinger for leukemia; therefore, we should cautiously conduct patient follow up and look out for the development of leukemia. At 1 year follow up after systemic chemotherapy, the patient had no evidence of cutaneous recurrence and development of systemic leukemia.
Figures and Tables
References
1. Mei-Yu H, George FM. Cutaneous lymphomas and leukemias. Lever's histopathology of the skin. 2009. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins;956–957.
2. King A. A case of chloroma. Month J Med. 1853. 17:97.
3. Rappaport H. Tumors of the hematopoietic system. Atlas of tumor pathology, section III, fascicle 8. 1965. Washington, DC: Armed Forces Institute of Pathology;241–247.
4. Burns A. . Observations of surgical anatomy in head and neck. 1811. London: Royce;364.
5. Eshghabadi M, Shojania AM, Carr I. Isolated granulocytic sarcoma: report of a case and review of the literature. J Clin Oncol. 1986. 4:912–917.

6. Shea B, Reddy V, Abbitt P, Benda R, Douglas V, Wingard J. Granulocytic sarcoma (chloroma) of the breast: a diagnostic dilemma and review of the literature. Breast J. 2004. 10:48–53.

7. Srinivasan B, Ethunandan M, Anand R, Hussein K, Ilankovan V. Granulocytic sarcoma of the lips: report of an unusual case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008. 105:e34–e36.

8. Byrd JC, Edenfield WJ, Shields DJ, Dawson NA. Extramedullary myeloid cell tumors in acute nonlymphocytic leukemia: a clinical review. J Clin Oncol. 1995. 13:1800–1816.

9. Eisenberg E, Peters ES, Krutchkoff DJ. Granulocytic sarcoma (chloroma) of the gingiva: report of a case. J Oral Maxillofac Surg. 1991. 49:1346–1350.

10. Fellbaum C, Hansmann ML. Immunohistochemical differential diagnosis of granulocytic sarcomas and malignant lymphomas on formalin-fixed material. Virchows Arch A Pathol Anat Histopathol. 1990. 416:351–355.

11. Amin KS, Ehsan A, McGuff HS, Albright SC. Minimally differentiated acute myelogenous leukemia (AML-M0) granulocytic sarcoma presenting in the oral cavity. Oral Oncol. 2002. 38:516–519.

12. Brunning RD, McKenna RW. Atlas of tumor pathology. Tumors of bone marrow, third series, fascicle 9. 1994. Washington, DC: Armed Forces Institute of Pathology;19–37.
13. Cankaya H, Ugras S, Dilek I. Head and neck granulocytic sarcoma with acute myeloid leukemia: three rare cases. Ear Nose Throat J. 2001. 80:224–226.

14. Imrie KR, Kovacs MJ, Selby D, Lipton J, Patterson BJ, Pantalony D, et al. Isolated chloroma: the effect of early antileukemic therapy. Ann Intern Med. 1995. 123:351–353.

15. Neiman RS, Barcos M, Berard C, Bonner H, Mann R, Rydell RE, et al. Granulocytic sarcoma: a clinicopathologic study of 61 biopsied cases. Cancer. 1981. 48:1426–1437.

16. Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: report of two cases and a review of 72 cases in the literature. Cancer. 2002. 94:1739–1746.

17. Shin SB, Lee DW, Lee JY, Cho BK. A case of acute myelocytic leukemia developed the cutaneous granulocytic sarcoma (chloroma) and leukemia cutis. Korean J Dermatol. 2000. 38:933–936.
18. Jang JW, Kwon SB, Kim DW, Jun JB, Chung SL. Cutaneous granulocytic sarcoma: a report of two cases. Korean J Dermatol. 2000. 38:1225–1229.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download